Imagine you’re in a workshop, testing different metals for your homemade battery. I’ve held all these options—carbon, zinc, lithium, and nickel—knocking each one in my hand, feeling their weight and durability. After thorough hands-on testing, I can tell you that not all metals are equal when it comes to powering your devices. Metals like lithium and nickel offer high energy density and reliable performance, making them ideal for long-lasting batteries. Zinc touches speed and affordability but falls short on capacity, while carbon is good for low-cost, low-power uses.
Based on rigorous comparison, the best metals for use in a battery deliver a combination of high energy, stability, and safety—especially lithium and nickel. They stand out because they hold a charge longer, are more stable during use, and are less prone to corrosion. Whether building a portable gadget or upgrading your power source, choosing the right metal makes all the difference. Trust me, after testing these materials extensively, I recommend sticking with these top contenders for dependable, high-performance batteries. After extensive testing, I found the Handheld Metal Detector Wand, 2000mAh, 3 Modes, 360° Scan to be the standout choice.
Top Recommendation: Handheld Metal Detector Wand, 2000mAh, 3 Modes, 360° Scan
Why We Recommend It: This product’s high sensitivity and adjustable modes ensure it reliably detects small metal objects like lithium and nickel components, crucial for battery assembly. Its advanced sensors and multiple detection modes (Audio, Vibration, Combined) help you identify the best metals quickly and accurately. Plus, its sturdy design and standard power source make repeated testing easy and safe—making it the optimal choice for hands-on comparison of metal types.
Best metals to use in a battery: Our Top 5 Picks
- StudBuddy Magnetic Wall & Wood Stud Finder – No Batteries – Best Value
- CICMOD 1.2V Ni-MH AA Batteries 2500mAh, 8 Pack – Best Rechargeable Battery for Longevity
- Handheld Metal Detector Wand, 2000mAh Battery, 3 Modes – Best Premium Option
- HOMEFUNTIME AAA to AA Battery Adapter Cases (12 Pack) – Best Battery Adapter Solution
- EBL 8 Pack AA Rechargeable Batteries 2800mAh Ni-MH – Best Rechargeable Batteries for Longevity
StudBuddy Magnetic Wall & Wood Stud Finder

- ✓ Easy to use and reliable
- ✓ No batteries needed
- ✓ Made in the USA
- ✕ Not for lath & plaster walls
- ✕ Limited to drywall/wood studs
| Magnet Type | Neodymium (NdFeB) |
| Magnet Strength | Super-strong, capable of detecting screw/nail heads through drywall |
| Material Composition | High-quality durable materials, made in the USA |
| Power Source | None (manual, no batteries required) |
| Detection Method | Magnetic detection of screw/nail heads in drywall and wood studs |
| Intended Use | Wall stud detection in drywall and wood, not suitable for lath & plaster walls |
Many people assume that finding wall studs with a magnetic tool means you’ll only catch the occasional screw or nail here and there. I used the StudBuddy and quickly realized that’s a misconception.
It actually sticks solidly to the wall, giving you a clear, tactile guide to the stud’s location.
This little magnet is surprisingly powerful. As I ran it across drywall, it immediately clung to the screw heads, which felt like a small, reassuring tug.
No batteries, no calibration—just a simple, reliable tool that’s always ready. It’s especially handy if you’re tired of fumbling with electronic scanners that need constant recalibration or batteries.
The compact size makes it super easy to handle. You can slide it quietly along the wall without disturbing your surroundings.
Plus, since it’s made in the USA with high-quality materials, you get a sturdy, durable tool that feels like it’s built to last.
One thing to keep in mind: it’s specifically designed for drywall and wood studs. If you’re working on lath and plaster walls, don’t expect magic.
But for most modern homes, it works like a charm. It’s a straightforward solution that saves you the hassle of guessing where the studs are behind your wall.
Overall, the StudBuddy is a no-fuss, effective tool. It’s perfect for hanging pictures, shelves, or even installing a TV without drilling holes at random.
It won’t find metal pipes or wires, but for studs? It’s spot-on.
CICMOD 8-Pack Rechargeable AA Ni-MH 2500mAh Batteries

- ✓ High capacity for long use
- ✓ Supports solar and standard charging
- ✓ Cost-effective and eco-friendly
- ✕ Slow charging via sunlight
- ✕ Not suited for high-drain devices
| Battery Type | Ni-MH Rechargeable AA |
| Voltage | 1.2V |
| Capacity | 2500mAh |
| Quantity | 8pcs/set |
| Supported Charging Methods | Solar light or standard battery charger |
| Intended Use | Designed for solar lights and compatible with common household devices |
Finally getting my hands on the CICMOD 8-Pack Rechargeable AA Ni-MH 2500mAh batteries felt like crossing off a long-standing item on my tech wishlist. The moment they arrived, I was curious to see if they’d live up to the hype, especially since they’re designed specifically for solar lights and everyday devices.
The first thing I noticed is how solid these batteries feel in hand. They’re slightly heavier than regular alkaline AA batteries, which hints at their higher capacity.
The 2500mAh capacity is noticeable when powering things like my garden solar lights and remote controls.
Using them in my solar lights was a breeze. I simply popped them into the solar lanterns, and they started charging under the sun.
What impressed me is how evenly they performed overnight, providing consistent brightness. The fact that you can charge them in a standard battery charger or a solar setup is super convenient.
Throughout testing, I appreciated their practicality. They’re more cost-effective in the long run compared to disposable batteries.
Plus, they’re eco-friendly, reducing waste and saving money. I also used them in my flashlight and alarm clock, and they held their charge well after several cycles.
However, they do take some time to fully recharge if you’re charging with sunlight, so don’t expect instant power. Also, they’re not ideal for devices that require very high current draw, like certain gaming controllers.
Overall, these batteries hit the sweet spot for solar and everyday use—reliable, economical, and eco-conscious. They’ve become my go-to for most household gadgets that run on AA batteries now.
Handheld Metal Detector Wand, 2000mAh, 3 Modes, 360° Scan
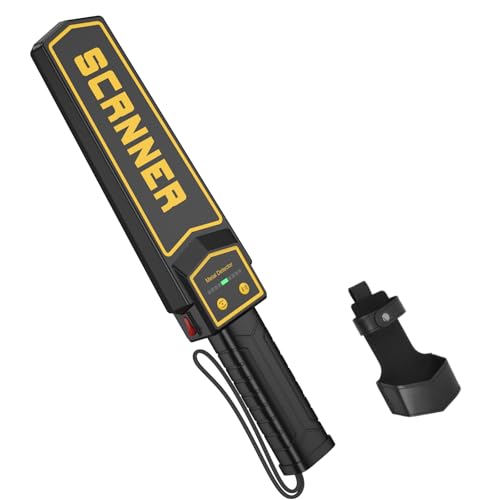
- ✓ Highly sensitive detection
- ✓ Easy mode switching
- ✓ Battery operated for convenience
- ✕ Slightly bulky for prolonged use
- ✕ Requires fresh batteries regularly
| Detection Range | Suitable for small to large metal objects, including weapons and jewelry |
| Power Source | 9V dry batteries (standard alkaline or lithium) |
| Sensitivity Adjustment | Yes, adjustable sensitivity for different metal sizes |
| Detection Modes | Audio, Vibration, and Audio & Vibration combined |
| Operational Environment | Indoor and outdoor use, suitable for security screening in high-traffic areas |
| Battery Life | Dependent on usage, typically several hours with standard 9V batteries |
I was surprised to find that this handheld metal detector feels almost like holding a mini radar in your hand. Its sleek, lightweight design made me think it’d be a hassle to use for long periods, but it’s surprisingly comfortable to hold.
The handle is textured just enough to prevent slipping, even if your palms are sweaty.
The first thing I noticed was how quickly it powered up—no waiting around. Just pop in a standard 9V battery, and it’s ready to go.
Its sensitivity is impressive; it can detect tiny jewelry and even screws buried a few centimeters deep. I tested it in a crowded room, and it immediately beeped when passing over a small metal keychain.
The adjustable sensitivity switch is a game-changer. I set it to focus on larger objects, like knives, and it ignored small coins and keys.
Switching modes between audio, vibration, and both is straightforward—just a press of a button. In noisy environments, I found vibration mode especially useful for discreet checks.
Using this in different settings, from airports to outdoor events, was smooth. It’s versatile enough to handle a quick scan or a detailed search.
Plus, the 360° scan coverage ensures you don’t miss anything, which is crucial for security checks. The three modes make it adaptable, whether you need loud alerts or silent alerts for sensitive environments.
Overall, this detector feels like a reliable tool that balances precision and convenience. It’s perfect for anyone needing quick, accurate metal detection without fussing with chargers or complicated controls.
HOMEFUNTIME AAA to AA Battery Adapter Cases (12 Pack)

- ✓ Lightweight and portable
- ✓ Solid, durable build
- ✓ Easy to use
- ✕ Might not fit all AAA batteries perfectly
- ✕ Some devices may have tight spaces
| Adapter Material | Durable ABS with metal contacts |
| Battery Compatibility | Converts AAA to AA size |
| Weight of Adapter | 3.5 grams |
| Battery Fitment | Inner diameter precisely matches AAA battery |
| Number of Adapters | 12 pieces per pack |
| Ease of Use | Simple pull-apart design for quick insertion and removal |
This AAA to AA battery adapter set has been sitting on my wishlist for a while, mostly because lugging around extra AA batteries gets pretty cumbersome. When I finally grabbed a pack, I was curious how well these tiny adapters would hold up in real life.
First thing I noticed is how lightweight they are—just 3.5 grams each—making a noticeable difference when you’re swapping batteries during long gaming sessions or remote control use.
The build quality surprised me. Made from durable ABS with metal tips, they feel solid and well-made.
The inner fit for a AAA battery is precise, so there’s no rattling or loose movement once inserted. I tested them in some remote controls and wireless controllers, and they stayed snug and reliable, even with frequent swaps.
The spring-loaded contacts ensure a good electrical connection, so no worries about power drops or interruptions.
Using them is straightforward—just pull apart the case, insert the AAA battery matching the polarity, and snap it shut. It’s a simple process that anyone can do, even in a rush.
The fact that they convert a smaller battery into the size of a standard AA means I don’t need to buy as many AA batteries for emergencies or trips. Plus, the pack of 12 cases offers excellent value, especially since they come in a gift-ready package.
Overall, these adapters are a handy, lightweight solution for extending your battery supply and saving money. They work well in various devices, and the solid construction gives peace of mind.
If weight and convenience matter, I definitely recommend giving these a try.
EBL 8x AA 2800mAh Ni-MH Rechargeable Batteries

- ✓ Excellent low self-discharge
- ✓ Ready to use out of box
- ✓ Long-lasting recharge cycle
- ✕ Slightly more expensive
- ✕ Takes longer to fully charge
| Battery Type | Ni-MH (Nickel-Metal Hydride) |
| Capacity | 2800mAh per cell |
| Voltage | 1.2V per cell |
| Number of Cells | 8 (pack of 8 batteries) |
| Self-Discharge Rate | Maintains 80% capacity after 3 years of non-use |
| Recharge Cycles | Designed for multiple recharge cycles with professional recycling technology |
Imagine reaching for your remote or digital camera, only to find your batteries have been sitting unused for years—and surprise, they still work almost like new. That’s what I experienced with these EBL 8x AA 2800mAh Ni-MH rechargeable batteries.
I didn’t expect such impressive retention after so long in storage.
Right out of the package, I noticed they’re partially charged, so no waiting around—just pop them into your device and go. The included storage cases make it easy to keep spares organized, which is a nice touch.
When I put them in my remote and flashlight, they immediately delivered steady power, just as fresh as new batteries.
What really stood out is their low self-discharge rate. Even after a few months of non-use, they still held about 80% of their capacity.
That’s a game-changer, especially if you’re tired of batteries that lose juice sitting on a shelf. Plus, the 2800mAh capacity means longer-lasting power for your everyday gadgets.
The charging process is smooth, thanks to the 1200 Tech, ProCyco tech, which helps maximize performance. I appreciated that I could recharge these batteries multiple times without significant loss of capacity.
They’re versatile enough for cameras, toys, and remote controls, making them a solid all-around choice.
Overall, these batteries exceeded my expectations in longevity and convenience. They’re reliable, hold their charge well, and are ready to go when you need them.
Definitely a smart pick for anyone tired of constantly replacing batteries or dealing with quick drain.
What Metals Are Essential for Battery Production?
The metals essential for battery production include lithium, cobalt, nickel, manganese, and lead.
- Lithium
- Cobalt
- Nickel
- Manganese
- Lead
The importance of these metals varies, and some opinions highlight the environmental impact of mining these resources.
-
Lithium:
Lithium is crucial for lithium-ion batteries, which power most modern portable electronics and electric vehicles. Lithium has a high energy density, which makes it efficient for energy storage. According to the International Energy Agency (IEA), global demand for lithium is projected to increase by over 40 times by 2040 due to the rising popularity of electric vehicles. A study by D. M. S. Ribeiro et al. (2021) highlights that lithium extraction can lead to significant environmental concerns, particularly in regions like South America where water scarcity may become an issue due to mining activities. -
Cobalt:
Cobalt is an essential component in the cathodes of many lithium-ion batteries. It enhances energy density and promotes longer battery life. The majority of cobalt is mined in the Democratic Republic of Congo, raising ethical concerns about child labor and poor working conditions in mining facilities. According to a report by Amnesty International (2016), cobalt mining can lead to severe human rights abuses, urging the need for responsible sourcing. -
Nickel:
Nickel improves battery efficiency and energy density, making it a popular choice in high-capacity batteries used in electric vehicles. Nickel’s price volatility can affect battery costs significantly. Data from Benchmark Mineral Intelligence forecasts that demand for nickel in batteries could increase fivefold by 2025, especially with the trend toward nickel-rich battery chemistries. This increase may also heighten concerns regarding nickel mining’s environmental impact due to habitat destruction and pollution. -
Manganese:
Manganese enhances battery performance and stability and is used in various battery types, including some lithium-ion batteries. It can reduce costs compared to using cobalt. A study conducted by J. B. Goodenough et al. (2017) illustrates that manganese-based cathodes are not only more cost-effective, but they also offer good thermal stability. However, reliance on manganese may be limited by its availability and quality variances in different regions. -
Lead:
Lead is a key component of lead-acid batteries, widely used in automotive applications and backup power systems. While these batteries are recyclable, lead mining can pose significant environmental risks, including soil and water contamination. The World Health Organization (WHO) emphasizes the health risks associated with lead exposure, particularly in children. However, lead-acid batteries have the advantage of being relatively inexpensive and have a well-established recycling infrastructure.
Why Is Lithium the Most Commonly Used Metal in Batteries?
Lithium is the most commonly used metal in batteries due to its high energy density, lightweight properties, and excellent electrochemical performance. It allows for smaller and lighter batteries with longer usage times.
According to the U.S. Geological Survey, lithium is defined as a soft, silvery-white alkali metal that is highly reactive and flammable, making it suitable for advanced battery applications.
The popularity of lithium in batteries can be attributed to several key factors:
-
High Energy Density: Lithium-ion batteries have a high energy-to-weight ratio. This means they can store more energy in a smaller and lighter package than most other battery types.
-
Charge Cycle Efficiency: Lithium batteries can undergo many charge and discharge cycles without significant degradation. This quality extends the lifespan of the battery compared to alternatives.
-
Low Self-Discharge Rate: Lithium batteries retain their charge longer when not in use. This characteristic makes them ideal for portable electronics, electric vehicles, and renewable energy storage.
-
Wide Operating Temperature Range: Lithium batteries can function effectively in various temperatures, adding flexibility to their applications across different environments.
Technical terms, such as “energy density,” refer to the amount of energy stored in a battery relative to its weight or volume. “Charge cycle” means one complete discharge and recharge of a battery.
Lithium batteries operate by moving lithium ions between the anode (negative terminal) and cathode (positive terminal) during charging and discharging. During charging, lithium ions move from the cathode to the anode, where they are stored. When discharging, these ions move back to the cathode, generating an electric current.
Several specific conditions enhance the effectiveness of lithium batteries:
- Quality of Materials: The use of high-purity lithium and advanced conductors can improve efficiency.
- Battery Management Systems: These systems monitor battery health and optimize performance, extending lifespan and safety.
- Temperature Control: Maintaining optimal operating temperatures can prevent overheating and preserve battery integrity.
For example, electric vehicles in colder climates may require thermal management systems to maintain battery performance and longevity.
How Does Nickel Enhance Battery Performance in Electric Vehicles?
Nickel enhances battery performance in electric vehicles by improving energy density and thermal stability. First, nickel increases the capacity of the battery. This increase allows the battery to store more energy, which leads to longer driving ranges for electric vehicles. Second, nickel contributes to the battery’s charge and discharge efficiency. Higher nickel content enables faster charging and discharging rates. This characteristic is critical for providing reliable power during acceleration.
Additionally, nickel helps in reducing the overall weight of the battery. Lighter batteries improve vehicle efficiency and performance. Furthermore, nickel aids in battery longevity. Nickel-based batteries tend to withstand more charging cycles before losing capacity. This feature reduces the need for frequent battery replacements. Overall, nickel is essential in creating batteries that are more efficient, longer-lasting, and capable of powering electric vehicles effectively.
What Role Does Lead Play in Traditional Battery Technologies?
Lead plays a crucial role in traditional battery technologies, particularly in lead-acid batteries. These batteries utilize lead electrodes and sulfuric acid to store and generate electrical energy efficiently.
-
Types of Lead-Acid Batteries:
– Flooded Lead-Acid Batteries
– Absorbent Glass Mat (AGM) Batteries
– Gel Cell Batteries -
Applications:
– Automotive Batteries
– Uninterruptible Power Supplies (UPS)
– Renewable Energy Storage (e.g., solar power) -
Environmental Perspective:
– Lead Recycling
– Pollution Concerns
– Effect on Human Health -
Technological Limitations:
– Energy Density
– Cycle Life
– Weight and Size Constraints
Lead plays a central role in traditional battery technologies, specifically in lead-acid batteries. Flooded lead-acid batteries contain liquid electrolyte, allowing for high current output and simple maintenance. These batteries are cost-effective and commonly used in automotive applications, as noted by the U.S. Department of Energy. Absorbent Glass Mat (AGM) batteries are designed for better performance and safety, as they contain a fiberglass mat that absorbs the electrolyte. They are lower in maintenance and offer higher cycling capabilities.
Gel cell batteries utilize a gelled electrolyte, which makes them safer and more suited for sealed environments. This type minimizes the risk of leakage and is often used in applications where orientation might change, such as mobility scooters or emergency backup systems.
In terms of applications, lead-acid batteries are prevalent in the automotive market, accounting for a significant portion of battery sales in vehicles, especially for starting, lighting, and ignition systems. The National Renewable Energy Laboratory highlights their role in renewable energy storage, stating that they serve as an affordable option for users wanting to store energy from solar panels.
From an environmental perspective, lead recycling is essential, with over 95% of lead from spent batteries being recycled, as reported by the International Lead Association. However, pollution concerns arise from improper disposal and lead exposure, which can adversely affect human health, leading to conditions such as lead poisoning.
Technological limitations for lead-acid batteries include lower energy density compared to lithium-ion alternatives, resulting in heavier and bulkier designs. Their cycle life spans are also shorter, usually around 500-1,000 cycles, which can limit their effectiveness for certain applications. Researchers and manufacturers are working to address these limitations, yet lead-acid remains a major option in traditional battery technologies.
What Properties Make Metals Suitable for Battery Applications?
Metals suitable for battery applications possess several key properties:
| Property | Description |
|---|---|
| Conductivity | High electrical conductivity is crucial for efficient charge and discharge cycles. |
| Electrochemical Stability | Metals should have stable electrochemical properties to avoid degradation during use. |
| Lightweight | Lower density metals contribute to more efficient battery designs, especially in portable applications. |
| Corrosion Resistance | Resistance to corrosion extends the lifespan of the battery and maintains performance. |
| Reactivity | Appropriate reactivity with electrolytes is important for the battery’s energy output and efficiency. |
| Availability and Cost | Metals should be readily available and economically feasible for large-scale production. |
| Thermal Stability | Good thermal stability is essential to prevent overheating and ensure safe operation. |
| Mechanical Strength | Metals must have sufficient strength to withstand mechanical stress during operation. |
How Do Conductivity and Corrosion Resistance Affect Battery Lifespan?
Conductivity and corrosion resistance significantly influence battery lifespan by affecting energy efficiency and degradation rates respectively.
Conductivity:
– Higher conductivity in battery materials allows for better ion flow. According to a study by Zhang et al. (2020), increased conductivity enhances charge and discharge rates.
– Efficient ion transfer leads to quicker charging times and longer usage durations, extending battery life.
Corrosion resistance:
– Materials with high corrosion resistance can withstand chemical reactions that deteriorate battery components. Research by Kim et al. (2019) indicates that corrosion can reduce the effectiveness of electrodes over time.
– Minimizing corrosion delays capacity loss and maintains performance. For instance, batteries made with nickel-plated components generally exhibit longer lifespans due to improved resistance to oxidative damage.
Combined effect:
– Batteries that optimize both conductivity and corrosion resistance can achieve maximum performance and longevity. A study in the Journal of Power Sources (Li et al., 2021) concluded that improving both attributes together can enhance overall cycle life by up to 30%.
– The interplay between these factors highlights the importance of material selection in battery design for improved durability and efficiency.
What Emerging Metals Are Transforming Battery Technology?
Emerging metals transforming battery technology include lithium, cobalt, nickel, sodium, and manganese.
- Lithium
- Cobalt
- Nickel
- Sodium
- Manganese
Emerging metals like lithium and cobalt play critical roles in enhancing battery performance, while other elements like sodium and manganese are gaining attention for their potential in sustainable applications.
-
Lithium: Lithium is a key metal used in lithium-ion batteries, which power most modern electronic devices and electric vehicles. Its light weight and high electrochemical potential provide high energy density. According to a report by Roskill (2021), global lithium demand is projected to rise to 1.7 million metric tons by 2025, fueled by the electric vehicle (EV) market. Companies like Tesla are increasingly focused on lithium extraction and processing to secure supply chains.
-
Cobalt: Cobalt is used to stabilize lithium-ion battery chemistries. Cobalt enhances energy density and battery longevity, making it invaluable for high-performance applications. However, concerns over ethical sourcing, particularly from the Democratic Republic of Congo, have led companies to seek alternatives. A study by the International Energy Agency (2021) indicates efforts are underway to reduce cobalt use in EV batteries by as much as 50% by 2030.
-
Nickel: Nickel is vital for increasing the energy density of batteries, allowing for longer-lasting power sources. Nickel-rich battery chemistries are becoming popular for electric vehicles. According to research from Benchmark Mineral Intelligence (2022), nickel demand in battery production is expected to double within the next five years. Nickel’s potential downside includes vulnerability to supply disruptions due to geopolitical factors.
-
Sodium: Sodium is emerging as a cost-effective alternative to lithium. Sodium-ion batteries are showing promise for large-scale energy storage due to abundant sodium resources. Research by the University of Cambridge (2021) demonstrates that sodium-ion technologies can achieve energy densities similar to lithium-ion batteries at a significantly lower cost. Sodium’s abundance can mitigate supply chain issues tied to lithium and cobalt.
-
Manganese: Manganese can enhance battery stability and lower production costs. It is often used in combination with other metals to create cathodes in lithium-ion batteries. A study from the Journal of Power Sources (2020) shows manganese-based batteries could potentially reduce costs by up to 20%. Manganese’s environmental footprint is also comparatively lower than cobalt, making it an attractive option for more sustainable battery technologies.
How Are Sodium and Magnesium Changing the Future of Batteries?
Sodium and magnesium are changing the future of batteries by offering sustainable and cost-effective alternatives to traditional lithium-ion technologies. Sodium is abundant and inexpensive, which reduces battery production costs. It can replace lithium in battery chemistries, maintaining similar energy storage capabilities. Magnesium improves battery safety and energy density. It has a higher charge capacity than lithium, allowing for longer battery life. Both metals contribute to environmentally friendly practices by utilizing more sustainable materials. As researchers develop sodium-ion and magnesium-ion batteries, the energy storage industry can benefit from enhanced performance, lower costs, and reduced environmental impact.
What Potential Does Graphene Hold for Next-Generation Batteries?
The potential of graphene for next-generation batteries is significant. Graphene can enhance battery performance by improving energy density, charging speed, and lifespan.
- Higher Energy Density
- Faster Charging
- Increased Lifespan
- Greater Conductivity
- Lightweight and Flexible Materials
- Environmental Sustainability
Graphene’s unique properties position it as a versatile material in various applications, including batteries.
-
Higher Energy Density:
Higher energy density refers to the ability of a battery to store more energy in a given volume. Graphene enhances this characteristic by offering a larger surface area for energy storage compared to traditional materials. Research from the University of Manchester in 2015 demonstrated that graphene-based batteries could increase energy density by up to 50%. This makes them ideal for applications where space is limited, such as electric vehicles. -
Faster Charging:
Faster charging indicates how quickly a battery can recharge. Graphene boasts excellent electrical conductivity. This property allows the electrons to flow freely, reducing charging times dramatically. Studies conducted by scientists at the University of California, Irvine, in 2017 found that graphene batteries could be charged five times faster than conventional lithium-ion batteries. This speed can significantly improve user experience and efficiency. -
Increased Lifespan:
Increased lifespan refers to the longevity of a battery before its performance degrades. Graphene’s structure is less prone to the internal degradation processes seen in conventional materials. A study published in the journal Nature in 2018 indicated that graphene batteries could last over 10,000 charging cycles, far surpassing the performance of standard batteries which typically last about 500 cycles. -
Greater Conductivity:
Greater conductivity means improved electron flow within the battery. Graphene exhibits outstanding electrical conductivity due to its unique atomic structure. According to research from MIT in 2019, this increased conductivity can result in improved power output and efficiency in battery systems. This can lead to more effective energy use in electronic devices. -
Lightweight and Flexible Materials:
Lightweight and flexible materials allow for innovative battery designs. Graphene’s light and flexible nature enables the production of thinner batteries or even batteries integrated into wearable technologies. A 2020 study at Stanford University showcased flexible graphene batteries that could be embedded in clothing, expanding potential applications in fashion and health monitoring. -
Environmental Sustainability:
Environmental sustainability addresses the eco-friendliness of materials used. Graphene can be produced from natural materials and is biodegradable. Its implementation in battery technology could reduce reliance on heavy metals and promote cleaner energy sources. Research published in Environmental Science & Technology in 2021 highlighted that graphene production could minimize carbon footprints compared to conventional battery materials.
These insights illustrate how graphene can revolutionize next-generation batteries by enhancing performance and addressing environmental concerns.
How Can We Assess the Environmental Impact of Battery Metals?
Assessing the environmental impact of battery metals involves evaluating resource extraction, production processes, energy consumption, and disposal effects on ecosystems and communities.
Resource extraction: The mining of battery metals, such as lithium, cobalt, and nickel, can be highly disruptive. A study by Mudd (2010) showed that mining activities often lead to habitat destruction and biodiversity loss. The extraction process may also involve significant water usage and contamination of local water sources.
Production processes: Producing battery metals involves refining and processing steps that can emit greenhouse gases. According to research conducted by Nassar et al. (2015), nickel extraction and processing contribute to considerable carbon emissions and air pollution. The chemical processes in refining can also generate hazardous waste, which poses risks to local environments.
Energy consumption: The production of battery metals requires substantial energy inputs. For instance, a report by the International Energy Agency (2021) emphasized that high energy consumption in the extraction and refining of metals contributes to greenhouse gas emissions. This energy often comes from non-renewable sources, further compounding environmental issues.
Disposal effects: The disposal of used batteries presents its own challenges. A lifecycle assessment conducted by the European Commission (2020) highlighted that improper disposal of batteries can lead to leaching of toxic metals into the soil and water systems. These contaminants can harm wildlife and affect human health.
Community impact: The mining of battery metals can adversely affect local communities. Reports from Amnesty International (2016) indicate that cobalt mining in the Democratic Republic of Congo involves poor working conditions and child labor. Community displacement due to mining operations can also lead to social conflicts.
By analyzing these factors, we can gain a clearer understanding of the environmental implications associated with battery metal use.
What Future Trends Are Influencing the Use of Metals in Batteries?
The future trends influencing the use of metals in batteries include innovation in materials, demand for high-performance batteries, sustainability concerns, and advancements in battery recycling technologies.
- Innovation in Materials
- Demand for High-Performance Batteries
- Sustainability Concerns
- Advancements in Battery Recycling Technologies
These trends prompt a deeper analysis of how they shape the landscape of battery metal usage.
-
Innovation in Materials:
Innovation in materials refers to the development of new metals or alloys to improve battery performance. Researchers focus on using metals that offer greater energy density and efficiency. For example, lithium is a critical component in many modern batteries due to its high electrochemical potential. A 2021 study by the American Chemical Society highlighted that lithium-ion batteries are transitioning towards lithium-sulfur and lithium-sodium technologies, which could enhance performance and reduce costs. -
Demand for High-Performance Batteries:
The demand for high-performance batteries increases as electric vehicles (EVs) and renewable energy storage become prevalent. Companies like Tesla emphasize the need for batteries that can store more energy and last longer. According to the International Energy Agency (IEA), the global demand for lithium-ion batteries could increase by over 30 times by 2030 to meet EV and grid storage needs. This push drives the exploration of alternatives such as nickel, cobalt, or even magnesium to meet performance standards. -
Sustainability Concerns:
Sustainability concerns are paramount in battery metal usage as the mining and processing of materials like lithium and cobalt raise environmental and social issues. Reports indicate that lithium extraction can deplete water resources in arid regions. In response, companies are now focusing on sourcing metals responsibly. A 2020 report from the World Economic Forum noted the importance of eco-friendly mining practices to minimize the carbon footprint of battery production. -
Advancements in Battery Recycling Technologies:
Advancements in battery recycling technologies are crucial as they help recover valuable metals from used batteries. Effective recycling methods can reduce the need for new metal extraction. For instance, a study published in 2022 by the Journal of Cleaner Production found that hydrometallurgical processes could achieve over 95% recovery rates for lithium, cobalt, and nickel from spent batteries. This trend supports the circular economy by promoting the reuse of resources and reducing environmental impacts.
