A battery in a circuit provides electrical energy by converting chemical energy. It has two electrodes (anode, cathode) and an electrolyte. When connected, electrons flow from the anode (-) to the cathode (+) via the circuit, powering devices. Electrolyte enables ion exchange, maintaining charge balance.
I’m excited to share my extensive knowledge of How Batteries Work in a Circuit with you today. Throughout my years in the battery industry, I’ve been fascinated by the clever science behind these little energy powerhouses. As an experienced professional, I can tell you that understanding how batteries work in a circuit is not only essential but also quite intriguing! In this guide, we’ll dive deep into the world of batteries, uncovering the science behind them, and exploring their impact on modern life. So, buckle up, my friends, and get ready to embark on an electrifying journey through the inner workings of batteries in circuits! Let’s get charged up!
The Basics of Electrochemical Batteries
Now that we’ve got a good introduction to the topic, let’s dive into the heart of the matter and explore the Basics of Electrochemical Batteries. Trust me, once you grasp these fundamentals, you’ll be well on your way to becoming a battery guru.
Components of a battery
Every battery, whether it’s the rechargeable lithium-ion battery in your smartphone or the classic AA alkaline battery powering your remote, has three essential components: the anode (negative terminal), the cathode (positive terminal), and the electrolyte.
The anode and cathode are the battery’s electrodes, which are made of different materials depending on the battery type. These electrodes are immersed in the electrolyte, a chemical medium that allows ions to move between the electrodes but blocks electrons’ direct passage.
Chemical reactions and charge development
Now, let’s talk about the exciting part: the chemical reactions happening inside the battery! These reactions drive the flow of electrons and create an electric current in the external circuit.
In general, at the anode, a chemical reaction called oxidation occurs, where the anode material loses electrons, releasing energy in the process. These freed electrons then flow through the external circuit (e.g., an electrical device) to the cathode. At the cathode, another chemical reaction, reduction, takes place. During reduction, the cathode material gains electrons, completing the electrical circuit.
For example, in a simple zinc-carbon battery, the oxidation reaction at the zinc anode can be represented as:
Zn → Zn^2+ + 2e^-
While the reduction reaction at the carbon rod cathode occurs as:
2MnO2 + 2e^- → Mn2O3 + OH^-
These reactions generate an electric potential, which powers the devices connected to the battery. Voilà! Now you know the magic behind those little energy powerhouses that keep our gadgets running!
In the next section, we’ll explore How Batteries Produce Electricity and the role of electric potential difference. You’ll be amazed at how simple yet fascinating these processes are!
How Batteries Produce Electricity
Now that you have a solid understanding of the components and chemical reactions inside a battery, it’s time to unravel the mystery of How Batteries Produce Electricity. Honestly, this is where the magic really happens!
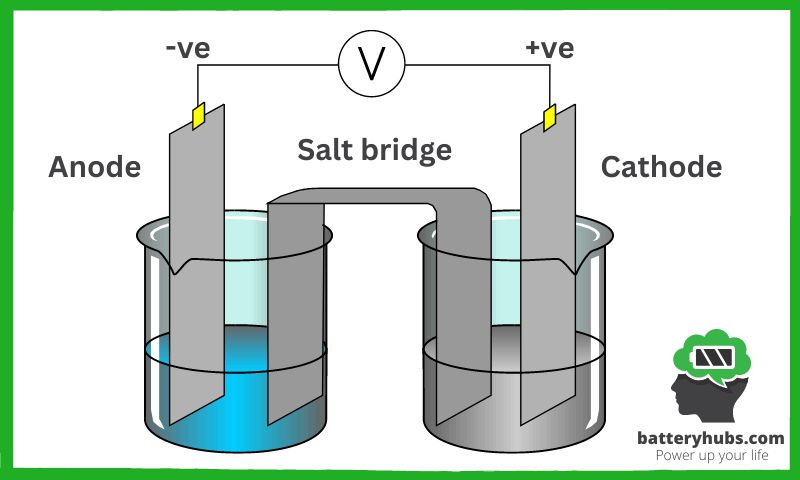
Conversion of chemical energy to electrical energy
As I mentioned earlier, batteries are like little wizards that convert chemical energy into electrical energy. This conversion occurs when the chemical reactions at the anode and cathode generate a flow of electrons, which we experience as an electric current.
When we connect a battery to an electrical device, these electrons rush through the external circuit, providing the power needed to operate the device. From flashlights to smartphones, it’s this flow of electrons that brings our electronic devices to life!
Electric potential difference (voltage) and its role
Now, let’s talk about the driving force behind this flow of electrons: the electric potential difference, or voltage. The voltage is essentially the “pressure” that pushes electrons from the anode to the cathode, powering the devices connected to the battery.
You can think of voltage as the difference in electric charge between the anode and cathode, created by the chemical reactions inside the battery. The greater the potential difference, the more “oomph” the battery has to power our devices.
Alessandro Volta, the genius who invented the first true battery, understood the importance of voltage. He stacked alternating layers of zinc and copper, separated by cardboard soaked in brine, to create the Voltaic Pile. The more layers he added, the higher the voltage and the more powerful the battery.
So there you have it, my friends! The secret sauce that powers our electronic devices is a clever combination of chemical reactions and electric potential differences. In the next section, we’ll explore How Batteries Work in Circuits and the importance of completing the loop for electrons to flow. Stay tuned for more electrifying insights!
So, we’ve explored how batteries produce electricity, and now it’s time to delve into the fascinating world of Connecting a Battery to an Electrical Circuit. This is where the battery’s stored energy is put to work, powering all the gadgets we know and love!
Connecting a Battery to an Electrical Circuit
When you connect a battery to an electrical circuit, the flow of electrons is what makes everything tick. Electrons move from the negative terminal (anode) to the positive terminal (cathode) through an external conductor, like a wire. This flow of electrons is what we call an electric current, which powers our devices and makes them functional.
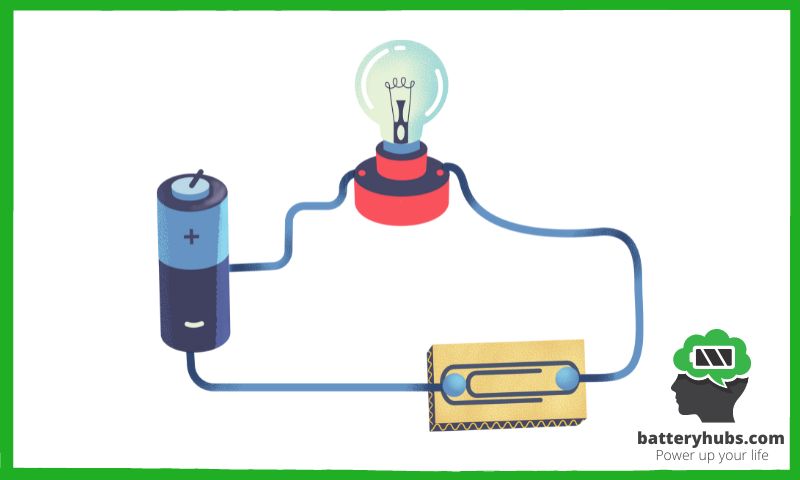
Example: D-cell battery powering a light bulb
Let me shine some light on this topic with a classic example: a D-cell battery powering a light bulb. When you connect the battery to the light bulb using wires, the electrons flow from the negative terminal through the wire, into the light bulb, and finally back to the positive terminal. This flow of electrons is what causes the filament inside the light bulb to glow, illuminating your surroundings.
Functions of electrical loads
In the world of electrical circuits, the term “load” refers to any component that consumes power. Light bulbs, motors, and electronic devices are all examples of loads. Loads are essential in electrical circuits because they transform the electrical energy provided by the battery into other forms of energy, such as light or motion.
When we connect a battery to an electrical circuit, the load determines how much current will flow through the circuit. This means the battery’s capacity to deliver power is directly related to the load’s resistance. So, the higher the resistance, the less current will flow, and the longer the battery will last.
Now that we’ve covered the basics of connecting a battery to an electrical circuit, it’s time to dive into some circuit essentials. Buckle up, because we’re about to explore the electrifying world of Closed and Open Circuits!
Circuit Essentials: Closed and Open Circuits
To power our devices, electrons need a complete path to travel through. In other words, we need a closed circuit for the electrons to flow from the battery’s negative terminal, through the electrical load, and back to the positive terminal. Without this complete path, there’s no flow of electrons, which means no electrical current, and ultimately, no power for our devices.
Switches are the unsung heroes of electrical circuits. They give us control over our devices by opening or closing the circuit, allowing or stopping the flow of electrons. When a switch is closed, the circuit is complete, and electrons can flow, powering our devices. When a switch is open, the circuit is broken, halting the flow of electrons and turning our devices off.
Example: An electrical circuit with two light bulbs and switches
Imagine a circuit with two light bulbs and two switches. When both switches are closed, the circuit is complete, and the electrons flow through both light bulbs, lighting them up. If we open one switch, the flow of electrons is interrupted, and the corresponding light bulb goes dark. The other light bulb remains illuminated because the electrons can still flow through the closed switch and the remaining closed part of the circuit.
In this example, we’ve seen how switches give us control over electrical devices by opening or closing the circuit. By mastering the art of closed and open circuits, we can harness the power of batteries to make our lives brighter, literally and figuratively!
Stay charged and ready for more exciting adventures in the world of batteries and electrical circuits!
After exploring the fascinating world of circuits, let’s take a trip back in time and delve into the history of batteries. Prepare yourself for a historical adventure as we uncover the story of the invention of the Electrochemical Cell!
A Glimpse into Battery History
The history of batteries wouldn’t be complete without mentioning the brilliant Alessandro Volta, an Italian scientist who invented the first real battery, known as the “Voltaic Pile,” in 1800. Believe it or not, this marked the beginning of the electrochemical cell’s story, and it revolutionized the way we generate and use electrical energy.
Volta’s invention came about after a heated debate with another scientist, Luigi Galvani, over the source of electricity in a frog’s leg experiment. You heard that right, folks! These two gentlemen were literally debating about electrified frog legs. Volta believed that the electricity originated from the metal electrodes, while Galvani argued that the electricity came from the frog’s tissues. To prove his point, Volta created the first true battery, and as they say, the rest is history.
Basic components and reactions in Volta’s battery
Volta’s battery consisted of alternating layers of zinc and copper discs, separated by pieces of cardboard soaked in saltwater. The zinc and copper acted as the negative and positive electrodes, while the saltwater served as the electrolyte, facilitating the flow of ions between the electrodes.
When connected in a closed circuit, a chemical reaction took place between the zinc and the electrolyte, causing the zinc to release electrons. These electrons then flowed through the external circuit to the copper electrode, creating an electric current. And just like that, the first-ever electrochemical cell was born, paving the way for future battery technology.
As we travel through the annals of battery history, it’s incredible to think that a simple experiment with frog legs led to the invention of the electrochemical cell. Today, we owe much of our modern electrical conveniences to Alessandro Volta and his groundbreaking invention. So, let’s raise our batteries to Volta and celebrate the remarkable history of these tiny powerhouses that have changed our lives!
Different Types of Batteries and Their Applications
Now that we’ve taken a nostalgic trip through the history of batteries, let’s get down to business and explore the various types of batteries available today. From powering your TV remote to jump-starting your car, batteries have come a long way since Volta’s time. So, buckle up, and let’s dive into the wonderful world of Different Types of Batteries and Their Applications!
A. Alkaline battery
Alkaline batteries are the most common type of disposable batteries, found in household items like remotes, toys, and flashlights. They have a higher energy density and longer shelf life compared to zinc-carbon batteries. Fun fact: alkaline batteries got their name because they use an alkaline electrolyte of potassium hydroxide, rather than the acidic ammonium chloride or zinc chloride found in zinc-carbon batteries.
B. Lead-acid battery
Lead-acid batteries have been around since the mid-1800s and are widely used in cars, trucks, and motorcycles for starting, lighting, and ignition. These batteries use lead oxide as the positive electrode, lead as the negative electrode, and sulfuric acid as the electrolyte. I like to think of them as the workhorses of the battery world, given their high power-to-weight ratio and ability to deliver large current surges.
C. Lithium battery
Lithium batteries are lightweight, high-energy batteries that can be found in devices like smartphones, laptops, and digital cameras. Their high energy density and low self-discharge rate make them perfect for devices that require a long battery life. But be careful, lithium batteries can be sensitive to high temperatures, so don’t leave your gadgets in a hot car!
D. Lithium-ion battery
Lithium-ion batteries are rechargeable and have a higher energy density than other rechargeable batteries, making them ideal for electric vehicles, portable electronics, and renewable energy systems. They use a lithium cobalt oxide or lithium manganese oxide positive electrode, a graphite negative electrode, and an organic electrolyte. Thanks to John B. Goodenough, the inventor of lithium-ion batteries, we can now enjoy longer-lasting devices!
E. Nickel-cadmium (NiCad) battery
NiCad batteries are rechargeable batteries that have been around since the early 1900s. They use nickel oxide hydroxide as the positive electrode, cadmium as the negative electrode, and potassium hydroxide as the electrolyte. NiCad batteries are known for their high discharge rate and ability to withstand extreme temperatures, making them popular in power tools and emergency lighting systems.
F. Zinc-carbon battery or standard carbon battery
Zinc-carbon batteries, also known as standard carbon batteries, are the OGs of disposable batteries. They use zinc as the negative electrode, manganese dioxide as the positive electrode, and an acidic electrolyte. Although they have a lower energy density and shorter shelf life compared to alkaline batteries, they’re still widely used in low-drain devices like wall clocks and remote controls.
From powering everyday devices to jump-starting cars, batteries have come a long way since their humble beginnings. It’s incredible to think about how many types of batteries have been developed over the years, each with its own unique characteristics and applications. So, the next time you replace a battery, take a moment to appreciate the amazing technology that keeps our world running!
Transformers and Long-Distance Electricity Transmission
As we’ve explored the fascinating world of batteries and their applications, it’s important to recognize the vital role that electricity transmission plays in our modern lives. So, without further ado, let’s switch gears and electrify our brains with some knowledge about Transformers and Long-Distance Electricity Transmission!
role of transformers in voltage regulation
Transformers are like the unsung heroes of the electrical world, quietly working behind the scenes to ensure the safe and efficient transmission of electricity. Their primary role is to regulate voltage levels between different parts of the electrical grid. By adjusting voltages, transformers help minimize power loss during transmission and ensure that the right voltage is delivered to homes, businesses, and other end users.
importance of high-voltage transmission lines
High-voltage transmission lines are the backbone of our electrical grid, allowing us to transmit electricity over long distances with minimal power loss. You see, when electricity travels through a conductor, it encounters resistance, which generates heat and leads to power loss. By increasing the voltage, we can reduce the current and, in turn, minimize power loss. So, high-voltage transmission lines are like the superhighways of electricity, efficiently delivering power to where it’s needed.
Stepping up and stepping down voltages for various uses
Transformers play a crucial role in stepping up and stepping down voltages for different parts of the electrical grid. At power plants, transformers step up the voltage, so it can be efficiently transmitted over long distances. Once the electricity reaches its destination, transformers step down the voltage to a level suitable for homes and businesses.
Imagine transformers as the traffic controllers of the electrical world, guiding the flow of electricity and ensuring it reaches its destination at the right voltage. Without transformers, we’d struggle to power our homes, businesses, and all those nifty devices we’ve come to rely on daily.
Practical Uses of Batteries in Circuits
After exploring the world of transformers and long-distance electricity transmission, it’s time to bring things back down to earth and discuss the Practical Uses of Batteries in Circuits. Let’s dive into the everyday applications of batteries and the value of understanding battery-powered circuits!
Everyday battery-powered devices
Batteries are the lifeblood of countless everyday devices, powering everything from remote controls to digital cameras. They bring convenience and portability to our lives, allowing us to operate gadgets without being tethered to an electrical outlet. Just think about it – without batteries, we’d be stuck with cords, cables, and a severe lack of mobility!
Some common battery-powered devices include:
- Flashlights
- Mobile phones
- Laptops
- Power tools
- Portable speakers
- Toys
- Wireless keyboards and mice
As you can see, batteries are essential to our daily lives. They keep our devices running, help us stay connected, and provide endless entertainment.
The value of understanding battery-powered circuits for DIY projects and troubleshooting
Understanding how battery-powered circuits work is a valuable skill, whether you’re a DIY enthusiast or just someone who likes to troubleshoot devices at home. When you know how batteries and circuits interact, you can:
- Diagnose and fix common problems with battery-powered devices, like loose connections or corroded terminals.
- Design and build your own custom battery-powered gadgets, like a homemade flashlight or a portable phone charger.
- Replace batteries safely and extend the life of your devices by selecting the right battery type and size.
- Enhance your understanding of electrical principles, like current, voltage, and resistance, which can be applied to a wide range of projects.
So there you have it – batteries and circuits are the unsung heroes of our modern lives, powering the devices that keep us connected, entertained, and productive. By understanding how they work, you can unlock a world of possibilities for DIY projects, troubleshooting, and more. So, get out there and explore the electrifying world of battery-powered circuits!
Conclusion
In this electrifying journey, we’ve explored the fascinating world of batteries, circuits, and their applications in our daily lives. From understanding the various types of batteries and their uses to diving into the role of transformers and voltage regulation, we’ve shed light on the power that lies within these small energy storage systems.
We’ve also touched on the importance of understanding battery-powered circuits, which opens doors for DIY projects and troubleshooting devices. The knowledge of batteries and circuits empowers us to tackle everyday challenges, make informed decisions about the devices we use, and even create our own custom gadgets.
As technology continues to advance, so too will battery technology, providing us with even more efficient and powerful solutions for our energy needs. So, stay curious, and keep exploring the ever-evolving world of batteries and circuits, as the potential for innovation is limitless!
We want to remind you of some essential resources available on our website to help you deepen your understanding of batteries and circuits. Our comprehensive guides on what is a battery and how batteries work will provide you with a solid foundation for your learning journey.
As you dive deeper into this electrifying topic, you may wonder how electricity travels from a battery or the form of energy that batteries store. Our articles are designed to answer these questions and more.
Rechargeable batteries are increasingly popular due to their sustainability and cost-effectiveness. Learn how rechargeable batteries work, how they get recharged, and if they keep their charge. For practical information, check out how long rechargeable batteries last when in use.
Finally, if you’re interested in specific battery types, such as the ubiquitous AA battery, discover how AA batteries work and explore the intriguing history behind why it is called a battery. These resources will empower you with knowledge and help you make informed decisions about the batteries you use in your everyday life. Happy learning!
FAQ
Q1: How do lithium-ion batteries work, and why are they popular in modern electronic devices?
A1: Lithium-ion batteries work through the movement of lithium ions between the positive and negative electrodes during charging and discharging. The electrolyte, usually a lithium salt in an organic solvent, allows for ion transport. These batteries are popular in modern electronic devices because they offer high energy density, long cycle life, low self-discharge rate, and are lightweight compared to other battery technologies.
Q2: What is the difference between primary and secondary batteries?
A2: Primary batteries, also known as non-rechargeable or disposable batteries, can only be used once. They convert chemical energy to electrical energy through irreversible electrochemical reactions. Common primary batteries include alkaline, zinc-carbon, and lithium batteries. Secondary batteries, on the other hand, are rechargeable and can be used multiple times. They store electrical energy by reversible electrochemical reactions, allowing them to be recharged when depleted. Examples of secondary batteries include lead-acid, lithium-ion, and nickel-cadmium batteries.
Q3: How can I prolong the life of my rechargeable batteries?
A3: Prolonging the life of rechargeable batteries involves proper care and usage habits. Some tips include:
- Avoid overcharging by using a smart charger that automatically stops when the battery is fully charged.
- Store batteries in a cool, dry place, and avoid exposing them to extreme temperatures.
- Allow a battery to cool down before charging or using it after a heavy discharge.
- Partially discharge and recharge the battery occasionally to prevent the “memory effect” in nickel-based batteries.
- Follow the manufacturer’s recommendations for charging, discharging, and storage.
Q4: What factors affect the capacity of a battery?
A4: The capacity of a battery, measured in ampere-hours (Ah) or watt-hours (Wh), depends on several factors, including:
- The battery’s chemistry, which determines its inherent energy density.
- The size and weight of the battery, with larger and heavier batteries generally having higher capacities.
- The discharge rate, as higher discharge rates can reduce the effective capacity.
- Temperature, as both high and low temperatures can negatively impact capacity.
- Age and usage, as batteries typically lose capacity over time and with repeated charge/discharge cycles.
Q5: Can I mix different types or brands of batteries in a device?
A5: Mixing different types or brands of batteries in a device is generally not recommended. This is because different battery chemistries have distinct voltage characteristics, discharge rates, and capacities. Mixing batteries can result in imbalanced discharging, reducing overall performance and possibly damaging the batteries or device. In some cases, it can even lead to leakage or rupture of the batteries. To ensure optimal performance and safety, use the same type and brand of batteries recommended by the device manufacturer.
