Rechargeable batteries work through electrochemical reactions, storing energy by converting electrical energy into chemical energy. Two main types: lithium-ion (Li-ion) & nickel-metal hydride (NiMH). Li-ion: higher energy density, longer lifespan, lightweight. NiMH: less energy density, eco-friendly, lower self-discharge. Charging reverses the reaction, restoring energy capacity.
I’ve been delving into the world of batteries for decades now, and I can’t even begin to tell you how many times I’ve been asked, “How does a rechargeable battery work?” After all, it’s a fascinating topic, especially when you consider how these little powerhouses have become such an integral part of our everyday lives.
So today, I’m excited to share my knowledge and experience with you, in the hopes of shedding some light on the inner workings of these remarkable energy storage devices. Let’s dive right in and unravel the mystery of rechargeable batteries, shall we?
Rechargeable Batteries: Chemistry and Components
Now that we’ve covered the basics, let’s move on to the heart of the matter – the chemistry and components of rechargeable batteries. I can’t wait to share with you the fantastic world of electrochemistry that makes these powerhouses tick.
Basic components of a rechargeable battery
Before we dive into the nitty-gritty of electrochemical reactions, let’s discuss the essential building blocks of rechargeable batteries. Brace yourselves for some electrifying info.
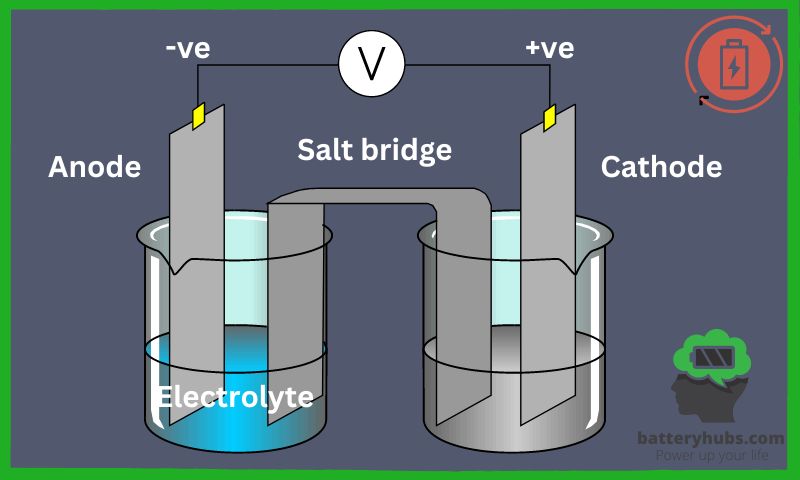
- Anode (negative electrode): The anode is the battery’s negative electrode, responsible for releasing electrons during discharge. A popular anode material is a graphite, allowing lithium ions to intercalate into its layers, creating a stable structure.
- Cathode (positive electrode): On the flip side, the cathode is the positive electrode, absorbing electrons during discharge. Cathodes are typically made from metal oxides, like lithium cobalt oxide (LiCoO₂), which is common in lithium-ion batteries.
- Electrolyte: The electrolyte is a medium that allows ions to move between the anode and cathode. In lithium-ion batteries, the electrolyte is usually a liquid, containing lithium salts dissolved in a solvent like ethylene carbonate or propylene carbonate.
- Separator: Last but not least, the separator is a critical component that keeps the anode and cathode apart, preventing short circuits. The separator is typically a porous polymer membrane, allowing ions to pass through while blocking electrons.
Now that we’ve covered the components let’s move on to the thrilling world of electrochemical reactions. Get ready for some chemistry magic.
The role of electrochemical reactions in rechargeable batteries
Rechargeable batteries rely on reversible electrochemical reactions to store and release energy. Here’s an example from a lithium-ion battery:
Discharge (energy release): Cathode: LiCoO₂ + e⁻ → Li⁺ + CoO₂ Anode: Li + xC₆ → LixC₆
Charging (energy storage): Cathode: Li⁺ + CoO₂ + e⁻ → LiCoO₂ Anode: LixC₆ → Li + xC₆
Comparing rechargeable and non-rechargeable batteries
| Feature | Rechargeable Batteries | Non-rechargeable Batteries |
|---|---|---|
| Reaction Type | Reversible | Irreversible |
| Lifespan | Can be charged hundreds of times | Single-use |
| Cost | Higher initial cost, lower long-term cost | Lower initial cost, higher long-term cost |
| Examples | Lithium-ion, nickel-metal hydride | Alkaline, zinc-carbon |
By understanding the chemistry and components of rechargeable batteries, we can appreciate their unique ability to power our devices time and time again.
As an experienced battery enthusiast, I’m constantly amazed by the incredible technology behind these energy-storing wonders. Let’s keep exploring and learning together.
Now that we’ve dived into the chemistry and components of rechargeable batteries, let’s explore the fascinating process of charging and discharging these energy-storing marvels. I’m sure you’ll find it electrifying.
Charging and Discharging Rechargeable Batteries
With a better understanding of their inner workings, it’s time to shed some light on how these batteries charge and discharge. Hold on tight, we’re going on a thrilling ride through the world of electrochemical reactions.
To charge a rechargeable battery, an external voltage is applied to the battery terminals, which drives the electrochemical reactions in reverse, restoring the battery’s energy capacity. The applied voltage must be higher than the battery’s initial voltage to facilitate this process.
Electrochemical reactions during charging and discharging
Here’s a closer look at the electrochemical reactions taking place in a lithium-ion battery during charging and discharging:
Discharge (energy release): Cathode: LiCoO₂ + e⁻ → Li⁺ + CoO₂ Anode: Li + xC₆ → LixC₆
Charging (energy storage): Cathode: Li⁺ + CoO₂ + e⁻ → LiCoO₂ Anode: LixC₆ → Li + xC₆
During discharge, the battery releases energy as lithium ions travel from the anode to the cathode, while electrons are released through the external circuit. When charging, the process is reversed, with lithium ions moving from the cathode back to the anode, and electrons being forced into the battery by the external voltage source.
The importance of battery chemistry in determining rechargeability
Battery chemistry plays a crucial role in determining whether a battery can be recharged or not. Rechargeable batteries, such as lithium-ion and nickel-metal hydride, rely on reversible electrochemical reactions that enable them to store and release energy multiple times.
In contrast, non-rechargeable batteries, like alkaline and zinc-carbon, use irreversible chemical reactions that can only release energy once. Once the reaction is complete, these batteries are considered “dead” and must be replaced.
As someone with tons of experience in the battery world, I’m always excited to share my knowledge about these incredible energy-storage devices. From their chemistry to the charging process, rechargeable batteries are truly the life force behind countless electronic devices that make our lives easier and more enjoyable. So, let’s continue to learn, laugh, and love these extraordinary power sources.
As we continue our electrifying journey through the world of rechargeable batteries, it’s time to dive into the different types and their applications. After all, variety is the spice of life, and the battery world is no exception.
Types and Applications of Rechargeable Batteries
Let’s take a closer look at some of the most common rechargeable battery types and their applications. Trust me, there’s a battery for just about anything you can imagine!
common rechargeable battery types
There are several types of rechargeable batteries, each with their own unique properties:
- Nickel-Cadmium (NiCd): Once the go-to rechargeable battery, NiCd has largely been replaced by more advanced options due to its lower energy density and environmental concerns.
- Nickel-Metal Hydride (NiMH): With higher energy density than NiCd and fewer environmental concerns, NiMH batteries are popular in applications like power tools and electric toothbrushes.
- Lithium-ion (Li-ion): Boasting high energy density and long cycle life, Li-ion batteries are the top choice for smartphones, laptops, and electric vehicles.
- Lithium-polymer (Li-Po): Known for their lightweight and flexible form factor, Li-Po batteries are commonly used in drones and wearable devices.
Comparison of rechargeable battery types and their advantages and disadvantages
| Battery Type | Advantages | Disadvantages |
|---|---|---|
| NiCd | Durable, low cost, good performance at low temperatures | Lower energy density, memory effect, toxic cadmium |
| NiMH | Higher energy density than NiCd, less prone to memory effect | Lower energy density than Li-ion, self-discharge |
| Li-ion | High energy density, long cycle life, low self-discharge | Can be sensitive to high temperatures, requires protection circuits |
| Li-Po | Lightweight, flexible form factor, can be made in various shapes | Lower energy density than Li-ion, more expensive |
Common applications of rechargeable batteries
Rechargeable batteries power a wide variety of devices, making our lives more convenient and enjoyable:
- NiCd: Emergency lighting, power tools, and two-way radios.
- NiMH: Power tools, electric toothbrushes, and digital cameras.
- Li-ion: Smartphones, laptops, electric vehicles, and power banks.
- Li-Po: Drones, wearable devices, and radio-controlled vehicles.
By now, you should have a solid understanding of the various rechargeable battery types and their applications. Whether you’re charging your smartphone or flying your drone, remember that behind every great device is an equally great rechargeable battery.
As our battery adventure continues, let’s talk about a topic that’s near and dear to every device lover’s heart: battery lifespan and charge retention. After all, we want our gadgets to stay powered up for as long as possible, right?
Lifespan, Charge Retention, and Factors Affecting Performance
In this section, we’ll explore how long rechargeable batteries last, how well they keep their charge, and the factors that can affect their performance. Let’s get charged up!
How long do rechargeable batteries last and do they keep their charge?
Rechargeable batteries come in all shapes and sizes, and their lifespans can vary depending on the battery chemistry and usage patterns. Some can be recharged hundreds of times before their capacity begins to drop significantly, while others can last even longer.
| Battery Type | Typical Cycle Life |
|---|---|
| NiCd | 500-1,000 cycles |
| NiMH | 300-1,000 cycles |
| Li-ion | 500-2,000 cycles |
| Li-Po | 300-500 cycles |
As for charge retention, most rechargeable batteries will self-discharge over time, with NiMH batteries having a higher rate of self-discharge than Li-ion batteries.
Factors affecting charge retention and battery lifespan
Several factors can impact a rechargeable battery’s lifespan and charge retention:
- Temperature: High temperatures can accelerate capacity loss and increase self-discharge rates.
- Overcharging: Charging a battery beyond its recommended voltage can cause permanent damage and reduce its lifespan.
- Deep discharge: Allowing a battery to discharge too deeply can negatively affect its lifespan.
- Usage patterns: Frequent, heavy use can lead to shorter battery life.
Memory effect and its impact on rechargeable batteries
The memory effect is a phenomenon that can occur in NiCd and, to a lesser extent, NiMH batteries. It’s when a battery “remembers” a smaller capacity due to repeated partial discharges, resulting in reduced performance. However, Li-ion and Li-Po batteries are not affected by the memory effect.
| Battery Type | Memory Effect? |
|---|---|
| NiCd | Yes |
| NiMH | Mild |
| Li-ion | No |
| Li-Po | No |
So there you have it, folks! A battery’s lifespan and charge retention can be influenced by various factors, and some battery chemistries are more susceptible to the memory effect than others. Always treat your batteries with care, and they’ll keep your devices powered up for many cycles to come.
As we continue our electrifying journey through the world of batteries, it’s time to discuss the care and maintenance of rechargeable batteries. After all, we want to make sure our trusty power sources live long, healthy lives, right?
Care and Maintenance of Rechargeable Batteries
In this section, we’ll share tips for prolonging battery life and optimizing performance, talk about signs of battery deterioration, and discuss the proper disposal and recycling of rechargeable batteries. Let’s give our batteries the TLC they deserve!
Tips for prolonging battery life and optimizing performance
Keeping your rechargeable batteries in tip-top shape isn’t rocket science. Follow these simple guidelines to ensure your batteries perform their best:
- Proper charging practices: Avoid overcharging and follow the manufacturer’s recommended charge time and voltage.
- Storage tips: Keep batteries in a cool, dry place away from direct sunlight and extreme temperatures.
- Device compatibility: Use the right battery type for your device, as specified in the user manual.
Signs of battery deterioration and when to replace
Even rechargeable batteries have a lifespan, and it’s essential to recognize the signs of deterioration. Here are some telltale signs that it’s time for a replacement:
- Shorter runtime: If your battery isn’t lasting as long as it used to, it’s likely losing capacity.
- Swelling or leakage: Physical changes like swelling or leakage indicate that a battery is damaged and should be replaced immediately.
- Inability to hold a charge: If your battery discharges quickly or won’t charge at all, it’s time for a new one.
Proper disposal and recycling of rechargeable batteries
Rechargeable batteries contain valuable materials that can be reused, so it’s essential to recycle them properly. Here’s how to dispose of your old batteries responsibly:
- Find a recycling facility: Many communities have designated recycling centers or drop-off points for rechargeable batteries. Check your local regulations to find a facility near you.
- Don’t throw batteries in the trash: Throwing batteries in the trash can lead to environmental damage and pose a risk to sanitation workers. Always recycle or dispose of batteries according to local guidelines.
And that’s a wrap on battery care and maintenance! By following these tips, you’ll not only prolong the life of your rechargeable batteries but also help protect the environment. Now, go forth and show your batteries some love.
As we reach the thrilling conclusion of our battery-powered adventure, it’s time to look to the future. In this section, we’ll explore emerging technologies and innovations in rechargeable batteries, discuss their potential applications in renewable energy and electric vehicles, and touch on sustainable practices and environmental concerns.
Future Developments and Impact of Rechargeable Batteries
The world of rechargeable batteries is ever-evolving, and we’re just getting started. From next-generation energy storage to reducing our environmental footprint, there’s no shortage of exciting developments on the horizon.
Emerging technologies and innovations in rechargeable batteries
Innovations in battery technology are happening at breakneck speed. Here are some game-changers to keep an eye on:
- Post-lithium-ion batteries: Researchers are exploring alternative materials, such as lithium-air and redox flow batteries, to improve energy density and life span.
- Solid-state electrolytes: Replacing liquid electrolytes with solid materials could lead to safer, more efficient batteries.
- Thin-film batteries: These lightweight, flexible batteries could revolutionize wearable and portable devices.
Potential applications in renewable energy and electric vehicles
Rechargeable batteries are playing a crucial role in the transition to sustainable energy and transportation. Here’s how:
- Energy storage: Advanced battery systems can store excess solar and wind energy, ensuring a steady power supply even when the sun isn’t shining or the wind isn’t blowing.
- Electric vehicles: Improvements in battery technology are making electric vehicles more accessible, with longer driving ranges and faster charging times.
- Smart grids: Rechargeable batteries can help balance power demand and supply, creating more efficient, reliable energy networks.
Sustainable Practices and environmental concerns
As we embrace the power of rechargeable batteries, it’s essential to consider their environmental impact. Here are some ways we can make battery use more sustainable:
- Recycling: Proper disposal and recycling of batteries help recover valuable materials and reduce waste.
- Eco-friendly materials: Developing batteries with sustainable materials can minimize their environmental impact.
- Efficiency: Maximizing battery life and optimizing charging practices can reduce the overall demand for new batteries.
That’s all, folks! Our journey through the world of rechargeable batteries has come to an end, but the future is bright. Keep an eye on these developments as they continue to shape the way we power our lives. Until next time, stay charged.
Conclusion
We’ve covered a lot of ground in our deep dive into the world of rechargeable batteries. From understanding the basics of how they work to exploring the different types, and even taking a peek into the future, we’ve left no stone unturned in our quest for battery knowledge.
As an active participant in this electrifying field, I hope you’ve found this journey both informative and engaging. Remember, the key to getting the most out of your rechargeable batteries is proper care and maintenance, as well as staying informed about the latest developments.
As technology continues to advance, rechargeable batteries will undoubtedly play an even more significant role in our daily lives, from powering our devices to enabling a more sustainable future. Stay charged, my friends, and until we meet again in the realm of battery-powered wonders, may the power be with you.
In case you’re craving even more battery knowledge, don’t worry, we’ve got you covered! We’ve got a treasure trove of resources to help you become a true battery connoisseur. For a deeper understanding of the fundamentals, check out our comprehensive guide on what a battery is. Once you’ve got that down, you can dive into how batteries work and explore the fascinating world of electricity travel in batteries.
Curious about the form of energy that batteries store? We’ve got you covered. And if you’re wondering about how batteries work in a circuit, we’ve got an article for that too! For those specifically interested in rechargeable batteries, you can learn about how they get recharged, whether they keep their charge, and how long they last when in use.
For those who want to focus on a specific type of battery, like the popular AA, check out our article on how AA batteries work. And finally, if you’ve ever wondered why it’s called a battery, we have a fun and informative piece to answer that question too.
So go ahead, dive into our extensive library of resources, and let your newfound battery wisdom shine bright!
