AA batteries work by converting stored chemical energy into electrical energy through a series of chemical reactions. The battery consists of a cathode, an anode, and an electrolyte. The cathode and anode are the positive and negative sides at either end of the battery. The chemical reactions within the battery cause a buildup of electrons at the anode, resulting in an electrical difference between the anode and the cathode.
In an alkaline AA battery, the anode is typically made of zinc, while manganese dioxide acts as the cathode. The electrolyte is a potassium hydroxide solution. The electrolyte oxidizes the anode’s powdered zinc, and the cathode’s manganese dioxide/carbon mix reacts with the oxidized zinc to produce electricity.
AA batteries are small cylindrical cells that can be made of alkaline, lithium, or Ni-MH (Nickel-Metal Hydride) compositions. They are commonly used in everyday devices such as remote controls, flashlights, and toys. The voltage of a fresh disposable alkaline AA battery is 1.5 volts, with a nominal capacity of around 2500 mAh (milliampere-hours).
It’s me, your trusty and energetic battery expert with years of experience under my belt. I remember the first time I held an AA battery in my hand, and it’s been a charged journey ever since.
Today, I am here to shed some light on the fascinating world of AA batteries. Did you know that they power more than 50% of portable electronic devices? That’s right! These little powerhouses have been silently fueling our lives for decades.
So, fasten your seatbelts, and let’s spark some curiosity as we dive into the electrifying details of how AA batteries work. Trust me, it’s going to be a shocking ride!
What are the main components of an AA battery?
Alright, folks! Let’s get down to business. To truly appreciate the magic of AA batteries, we need to take a closer look at their core components. You’ll find that it’s a trio of power players working together to create electricity: the cathode, anode, and electrolyte. Let’s explore each of these components in detail.
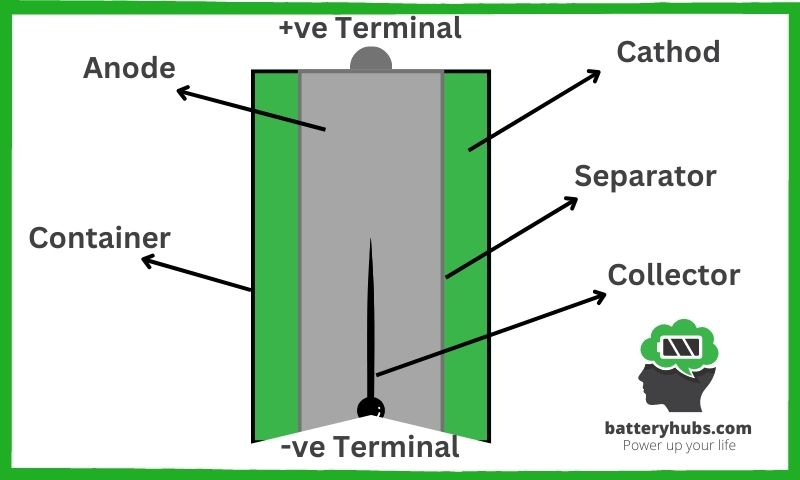
- Cathode (Positive Electrode): The cathode is the positive side of the battery. Typically, it’s made of manganese dioxide, which is a vital player in the whole electricity production game.
- Anode (Negative Electrode): Meet the battery’s negative side, the anode. Zinc is usually the material of choice for this electrode. You might say that the anode is the life of the party – it’s where all the action begins!
- Electrolyte: Acting as a referee between the cathode and anode, the electrolyte is a crucial component. It’s responsible for controlling the flow of ions during the discharge process. In most AA batteries, potassium hydroxide serves as the electrolyte.
Now that we’ve met our starring cast, let’s see how they come together to produce electricity:
- Anode: The anode is where the oxidation process occurs. In simple terms, it gives up electrons to generate a current.
- Cathode: The cathode’s job is to accept the electrons given up by the anode, which allows for the reduction process to take place.
- Electrolyte: The electrolyte acts as the battery’s traffic cop, ensuring that ions flow between the anode and cathode smoothly.
This harmonious relationship between the cathode, anode, and electrolyte is what brings an AA battery to life. As Thomas Edison once said, “The value of an idea lies in the using of it.” Similarly, the value of an AA battery lies in the successful collaboration between its components.
Now that we’ve delved into the heart of an AA battery, we can better appreciate the power they bring to our everyday lives. But don’t just take my word for it – let this newfound knowledge energize your curiosity and appreciation for these portable powerhouses!
How do chemical reactions generate electricity in AA batteries?
You might be wondering, “Hey, how does all this chemistry stuff actually create electricity?” Well, wonder no more! I’m about to electrify your understanding of how AA batteries work their magic. We’ll explore the electron buildup at the anode, the flow of electrons from the anode to the cathode, and the electrical difference between the two.
Remember how we talked about the anode being the life of the party? Here’s where things get interesting. During the discharge process, a chemical reaction causes the anode to oxidize, essentially donating electrons. This leads to a buildup of electrons at the anode, which creates an electrical potential.
Now that we’ve got all these electrons hanging out at the anode, they’re itching to party on at the cathode. When you connect a battery to a device, you’re creating a pathway for these electrons to flow from the anode to the cathode. This flow of electrons, my friends, is called an electric current.
“The battery is a wonderful device because it allows you to store energy in chemical form and then release it as electrical energy when needed.” – Prof. Donald Sadoway, MIT
The buildup of electrons at the anode results in a difference in electrical potential between the anode and cathode. This difference is what we call voltage. In a typical AA battery, the voltage is around 1.5 volts. It’s this voltage that ultimately drives the electric current through the device, powering it up.
| Anode (Negative) | Cathode (Positive) | Voltage |
|---|---|---|
| Electron buildup | Electron receiver | 1.5V |
What happens when an AA battery is discharged?

Now that we’ve uncovered the secret to generating electricity in AA batteries, let’s dive into the final stages of a battery’s life – when it’s discharged. We’ll explore the changes that happen inside the battery and how it affects its performance.
As the battery discharges, the chemical reactions inside it continue to produce electricity. However, over time, the reactants start to get used up, and the reaction rate slows down. Eventually, the battery can no longer produce enough voltage to power your device. That’s when we know it’s time for a replacement or a recharge!
As I mentioned earlier, a fresh AA battery typically provides around 1.5 volts. However, as the battery discharges, its voltage begins to drop. Most devices can tolerate a minor voltage drop, but if it falls below a certain threshold, it won’t operate properly. That’s why it’s important to keep an eye on your batteries and replace or recharge them when necessary.
So, how do we know when our AA battery has reached the end of its life? Here are a few telltale signs:
- Device malfunction: Your device may stop working or function erratically.
- Low voltage: You can measure the battery’s voltage with a multimeter, and if it’s significantly below 1.5 volts, it’s time for a change.
- Battery leakage: In extreme cases, a discharged battery may leak electrolytes, which can be corrosive and harmful.
| Fresh AA Battery | Discharged AA Battery |
|---|---|
| 1.5V | Below 1.5V |
| Steady voltage | Voltage drop |
| Device functions | Device malfunctions |
Rechargeable vs. Non-rechargeable AA Batteries
Now that we’ve explored the life of an AA battery, let’s talk about rechargeable and non-rechargeable batteries. After all, not all AA batteries are created equal! We’ll delve into the differences and the pros and cons of each type.
Rechargeable AA Batteries
Rechargeable AA batteries are designed to be recharged and reused multiple times, which can be a huge money saver in the long run. They come in various chemistries, including nickel-metal hydride (NiMH), nickel-cadmium (NiCd), and lithium-ion (Li-ion).
Pros:
- Environmentally friendly: Fewer batteries end up in landfills since they can be reused.
- Cost-effective: Over time, you’ll save money by recharging the same batteries rather than buying new ones.
- High capacity: Rechargeable batteries often have higher capacities, which means longer runtimes for your devices.
Cons:
- Higher initial cost: Rechargeable batteries tend to be more expensive upfront, although long-term savings make up for it.
- Performance degradation: Over time, rechargeable batteries may lose their ability to hold a charge or have reduced capacity.
- Require a charger: You’ll need a compatible charger to recharge the batteries.
Non-rechargeable AA Batteries
Non-rechargeable AA batteries, also known as primary batteries or single-use batteries, are designed to be used once and discarded after they’re depleted. The most common chemistry for non-rechargeable AA batteries is alkaline.
Pros:
- Lower initial cost: They are generally cheaper than rechargeable batteries, making them an attractive option for infrequent battery users.
- Long shelf life: Non-rechargeable batteries can be stored for several years without losing much of their charge.
- Ready to use: You don’t need a charger or any additional equipment to use non-rechargeable batteries.
Cons:
- Not eco-friendly: Since they’re single-use, non-rechargeable batteries contribute to electronic waste.
- More expensive over time: Constantly buying new batteries adds up in the long run.
| Rechargeable AA Batteries | Non-rechargeable AA Batteries |
|---|---|
| Eco-friendly | Not eco-friendly |
| Cost-effective | Lower initial cost |
| High capacity | Long shelf life |
| Require a charger | Ready to use |
Tips for Extending AA Battery Life
Alright, my fellow battery enthusiasts, let’s wrap things up with some practical tips to help you get the most out of your AA batteries. After all, nobody likes changing batteries more often than necessary, right?
A. Store Batteries Properly
Storing your batteries correctly can significantly extend their shelf life. Keep them in a cool, dry place, away from direct sunlight or extreme temperatures. Also, avoid storing batteries in a high-humidity environment, as moisture can lead to corrosion and leakage.
B. Remove Batteries from Unused Devices
If you’re not going to use a device for an extended period, it’s best to remove the batteries. This prevents potential leakage, which can damage the device and make the batteries unusable.
C. Use the Right Batteries for Your Devices
Some devices require specific battery types for optimal performance. Always check the manufacturer’s guidelines and use the recommended battery type for your device. For instance, high-drain devices like digital cameras might perform better with lithium or NiMH batteries, while low-drain devices like remote controls can use alkaline batteries.
D. Charge Rechargeable Batteries Correctly
To maximize the lifespan of your rechargeable batteries, follow the charging instructions provided by the manufacturer. Avoid overcharging, as it can damage the batteries and reduce their capacity. Also, it’s a good idea to invest in a quality charger with safety features like overcharge protection and smart charging technology.
E. Mix and Match with Caution
When using multiple batteries in a device, try to use batteries of the same type, brand, and age. Mixing old and new batteries or different chemistries can lead to uneven discharge rates and even damage your device.
Conclusion
And there you have it, folks! We’ve dived deep into the world of AA batteries, exploring their components, the chemical reactions that produce electricity, and the differences between rechargeable and non-rechargeable options. We’ve also shared some valuable tips to help you extend your battery life.
Remember, no matter which type of AA battery you choose, always use and dispose of them responsibly. Now, armed with your newfound knowledge, you can make informed decisions about the batteries you use and enjoy longer-lasting, more efficient power for your devices. Happy powering!
In our quest to become battery connoisseurs, we’ve only just scratched the surface. For more in-depth information on batteries and their inner workings, be sure to check out our other articles. Learn about the different types of batteries, dive deeper into how batteries work, and explore the fascinating world of electricity traveling from a battery.
Discover the form of energy that batteries store and how batteries work in a circuit. Uncover the mysteries of rechargeable batteries, how they get recharged, and whether they keep their charge. Find out how long rechargeable batteries last when in use and finally, learn why it is called a battery in the first place.
By exploring our extensive library of resources, you’ll become a true battery aficionado, ready to tackle any power-related challenge with confidence. Happy reading!
FAQ
What Are The Main Chemical Reactions In An AA Battery?
In an alkaline AA battery, the anode is typically made of zinc, while manganese dioxide serves as the cathode. The electrolyte within the battery oxidizes the zinc anode, and the manganese dioxide/carbon mix at the cathode reacts with the oxidized zinc to produce electricity.
Do AA Batteries Lose Power When Not In Use?
Yes, AA batteries gradually lose power when not in use due to self-discharge, which is a slow chemical reaction that occurs inside the battery, even when it’s not connected to a circuit. The rate of self-discharge depends on the type of AA battery and storage conditions.
What Is The Average Capacity Of An Alkaline AA Battery?
The average capacity of an alkaline AA battery is approximately 2,500 milliampere-hours (mAh). This figure, however, can vary depending on the brand, quality, and operating conditions.
How Do Rechargeable AA Batteries Work, And How Are They Different From Non-Rechargeable Ones?
Rechargeable AA batteries, such as nickel-metal hydride (NiMH) or lithium-ion (Li-ion) batteries, work by allowing their chemical reactions to be reversed during charging, restoring their capacity to provide power. In contrast, non-rechargeable batteries, like alkaline AA batteries, have chemical reactions that cannot be reversed, meaning they can only be used once.
