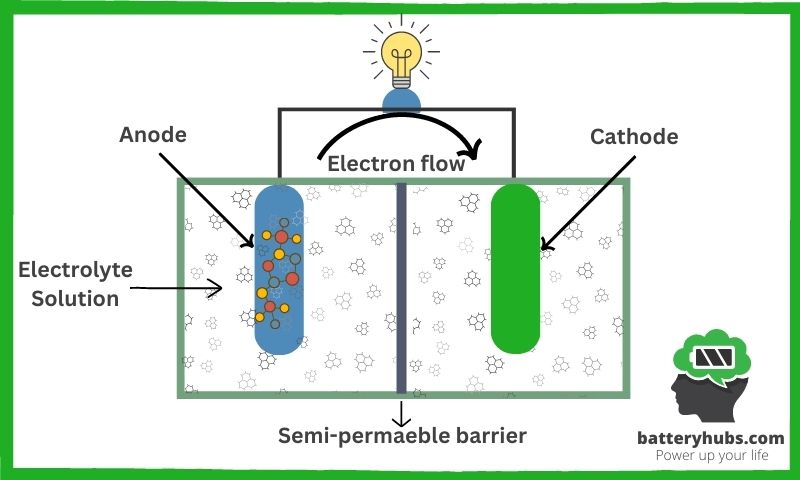
Batteries work by converting chemical energy into electrical energy. They consist of 3 main components: anode (negative), cathode (positive), and electrolyte. During discharge, chemical reactions occur at both electrodes, releasing electrons at the anode, which flow through a circuit, providing power. The electrolyte allows ions to move between the electrodes, maintaining charge balance.
Today I’ll be exploring the inner workings of batteries, what they are made of, and how they are able to store and transfer energy.
So whether you’re curious about the science behind batteries or just need a refresher on the basics, this blog post has something for everyone. Let’s get started!
What Is The Form Of Energy That Batteries Store Energy As?
Batteries store energy as chemical potential energy, which is a form of energy derived from the arrangement and position of atoms and molecules. This type of energy is not only found in batteries, but is used in many everyday energy sources such as fuel cells, solar cells, and even food!
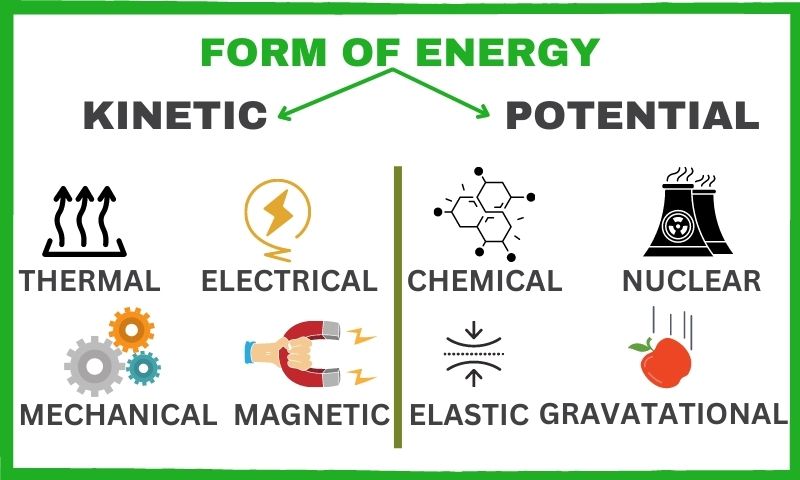
Chemical potential energy is the energy that exists between the bonds of atoms and molecules. When atoms or molecules are moved or rearranged, the potential energy is released, which is why it is as useful as an energy source.
Moreover, the chemical potential energy stored in a battery is converted into electrical energy which powers devices. This is done by the movement of electrons from one terminal to the other. This is what happens when you charge and discharge a battery.
On the other hand, the amount of energy stored in a battery is measured in watt-hours, which is the amount of energy you get when one watt of power is used for one hour. This is why it is important to buy the right type of battery for the device you are using.
Batteries store energy as chemical potential energy, which is derived from the arrangement and position of atoms and molecules. This energy is then converted into electrical energy which powers our devices. The amount of energy stored in a battery is measured in watt-hours, so it is important to buy the right type of battery for the device you are using.
How Does Electricity Travel From A Battery?
Electricity travels from a battery when a wire is connected to it. The battery redistributes the charges in the wire, creating an electric field that runs from the positive terminal to the negative terminal. The electron will then flow in the direction opposite to the electric field, allowing it to move from the positive to the negative terminal.
The battery is able to do this work due to the chemical reactions that take place inside it, resulting in a non-conservative force that creates a potential difference known as the electro-motive force.
Therefore, electricity will flow in the opposite direction as the electron, allowing it to travel from the battery.
How Batteries Work: Step-by-Step Process
Now I will discuss the chemistry and components of batteries and how they produce energy. I will explain the chemical process behind batteries and the individual parts of a battery and their function. Before going to step by step procedure about how does battery works, you need to know about
- What is the chemical process of a battery?
- Parts of battery and its function
Let’s get started.
What is the chemical process of a battery?
Examining the chemical process of a battery requires a look into the oxidation and reduction reactions, battery acid composition, transfer of electrons, and reactions to produce electricity.
- Oxidation and Reduction in Battery Process
Batteries are an essential part of many of our everyday devices. They are used to power everything from cars to computers and phones, but how do they operate? The answer lies in the chemical process known as oxidation and reduction.
Oxidation and reduction are two types of electrochemical reactions, which involve the transfer of electrons between two chemical species. Oxidation occurs when a species loses one or more electrons, while reduction occurs when a species gains electrons. An example of a reaction involving oxidation and reduction is the reaction between sulfuric acid and lead. In this reaction, the sulfuric acid (H2SO4) loses electrons, resulting in the formation of lead sulfate (PbSO4).
The electrochemical reaction between sulfuric acid and lead is an integral part of the battery process, as it enables the transfer of electrons from one chemical species to another. This process generates electricity, which is then used to power our devices.
In conclusion, oxidation and reduction are the two types of electrochemical reactions that occur within a battery. These reactions enable the transfer of electrons, which in turn produces electricity. Understanding the chemical process of a battery is essential to understanding how our devices operate.
- Battery Acid Composition
Battery acid is the common name for sulfuric acid (US) or sulphuric acid (UK). It is composed of hydrogen and sulfur with the chemical formula H2SO4. Battery acid is used in lead-acid batteries, and the concentration of sulfuric acid in water varies between 29% and 32%, or between 4.2 mol/L and 5.0 mol/L.
When a battery is in use, electrochemical reactions called oxidation and reduction occur. This process involves the exchange of electrons between chemical species. Oxidation is the loss of electrons, while reduction is the gain of electrons. In order to produce electricity, these reactions need to be arranged in order to allow electrons to flow through a wire. One of the reactions that can be used is 2Ag+(aq)+Cu(s)→2Ag(s)+Cu2+(aq).
- Transfer of Electrons
Batteries are useful everyday devices that allow us to power our electronics and other devices. But what is the chemical process that enables them to work? The key to understanding the process of a battery is to look at the transfer of electrons. This occurs through a process known as oxidation and reduction. In an oxidation reaction, electrons are transferred away from a particular chemical species, while in a reduction reaction, electrons are received by the same species.
A typical lead-acid battery is composed of two chemical species, lead and sulfuric acid. The sulfuric acid serves as the electrolyte and allows electrons to move between the two species. The oxidation reaction occurs when electrons are transferred away from the lead, while the reduction reaction occurs when electrons are received by the lead. This process results in the chemical energy of the battery being converted into electrical energy, which can then be used to power devices.
In order for the battery to work, the concentration of sulfuric acid in the water must be between 29% and 32%, or between 4.2 mol/L and 5.0 mol/L. This provides the necessary environment for the oxidation and reduction reactions to occur. The electrons that are transferred between lead and sulfuric acid are then used to create a circuit that allows electricity to flow through a wire.
The transfer of electrons is an essential part of battery operation and is the chemical process that enables batteries to power our devices. By understanding how oxidation and reduction reactions occur, we can better understand the inner workings of batteries and how they produce energy.
- Reactions to Produce Electricity
Batteries are an essential part of our everyday life and understanding the chemical process behind them is important for anyone who wants to make the most of their device.
The electrochemical reactions occurring inside batteries involve the transfer of electrons between chemical species. Oxidation and reduction are two of these reactions. Oxidation is the loss of electrons and reduction is the gain of electrons.
Lead-acid batteries use sulfuric acid, which has a concentration of 29-32% or 4.2 to 5.0 mol/L. To produce electricity, electrons need to flow through a wire, which can be achieved with a reaction like 2Ag+(aq)+Cu(s)→2Ag(s)+Cu2+(aq).
This reaction will allow the electrons to flow and create electricity.
Parts of the battery and its function
Let’s explore how each component functions within the overall battery system.
- Anode – Negative Side of Battery
The anode is the negative side of a battery, which acts as the source of electrons. It is typically made of a metal such as zinc or aluminum, which serves as the electron donor. The anode is where the oxidation reaction occurs, releasing electrons into the external circuit.
As electrons flow through the circuit, they are used to power the device. When the battery is depleted, the anode is oxidized and the electrons are no longer available for use.
This process can be reversed by providing energy to the battery, typically in the form of electrical current, which can cause the anode to be reduced.
As the anode is reduced, electrons are released back into the circuit, allowing the battery to be recharged.
- Cathode – Positive Side of Battery
The Cathode is the positive electrode in a battery and is responsible for accepting electrons from the external circuit.
It is composed of an oxidation-reduction couple made up of an oxidizing agent and a reducing agent and is connected to the anode through an electrolyte solution.
Consequently, the Cathode is the source of positive ions during the electrochemical reaction and helps power the battery.
Additionally, the anode serves as the source of negative ions during the reaction. In short, the Cathode is the positive side of the battery and is essential for its functioning.
- Electrolyte – Chemical Paste of battery
The electrolyte of a battery is a key component that allows it to function. It is typically a paste-like substance or a liquid, depending on the type of battery.
Its purpose is to transport positive ions between the anode and cathode terminals. The paste typically consists of lead oxide, which creates lead dioxide and sponge lead.
Additionally, Potassium hydroxide is the electrolyte in common household alkaline batteries, while lithium hexafluorophosphate (LiPF6) is the most common electrolyte in lithium batteries.
Ultimately, the electrolyte in a battery plays an essential role in providing the battery with its power.
- Container – Holding the Battery
A battery container is an essential component of a battery, as it holds the cell’s ingredients together to form the cathode, a part of the electrochemical reaction. Generally, battery containers are made from steel and are rated to withstand temperatures from 80-100°C (176-212°F).
However, the materials inside the container can be hazardous and can damage the environment if not disposed of properly. If placed in landfills, the toxic materials can leak into the soil and contaminate water sources.
Therefore, it is important to take appropriate safety measures when handling battery containers.
- Separator – Keeping Anode and Cathode Apart
A separator is a key component of any battery, as its primary purpose is to keep the anode and cathode from coming into direct contact with each other.
At the same time, it must be porous enough to allow the transport of ionic charge carriers that are necessary for the passage of current in an electrochemical cell.
Finding the right balance between mechanical robustness and porosity/transport properties is a challenge when designing a safe battery separator.
- Electrodes – Generating Electricity
Electrodes are an essential part of a battery, necessary for generating electricity. The anode, or negative electrode, releases electrons to the external circuit and oxidizes during the electrochemical reaction, while the cathode or the positive electrode, acquires electrons from the external circuit and is reduced during the electrochemical reaction.
Moreover, the electrodes are composed of atoms of a conducting material; for instance, in an alkaline battery, the anode is made of zinc, and manganese dioxide is used as the cathode. Ions in the electrolyte between and inside the electrodes complete the circuit, allowing for the flow of electricity.
- Collector – Capturing Electrical Output
The current collector is a critical component in a battery, providing a conduction path for electrons to be transferred from the electrochemical reaction to the external part without any reaction in the process.
Copper and aluminum are the most common current collectors for cathode and anode respectively. Copper, specifically in the form of a foil with a thickness of 5-12 micrometers, is the preferred material for the anode current collector due to its low resistivity and excellent electrochemical stability up to 3 volts.
Step By Step Process On: How Does Battery Work
Now that we have an understanding of the basic principles of how a battery works, let’s take a closer look at the step-by-step process.
Step-01:
A lithium-ion battery is a chemical machine that stores energy. To understand how it works, let’s start with the most fundamental element – hydrogen. Hydrogen is composed of a nucleus (made of 1 proton) and an electron orbiting the nucleus. The electron carries a negative charge and moves rapidly around the nucleus.
Step-02:
When a second proton is added to the hydrogen atom, it creates helium. It also attracts another electron to balance out the charge. The first shell of the atom has a limit of two electrons, so the two electrons share the existing electron shell. Helium is less reactive than hydrogen because the attractive force of the two protons in the nucleus is at its maximum compared to the first electron shell.
Step-03:
Adding one more proton creates the third lightest element on the periodic table – lithium. This creates the next electron shell which contains a lone electron. This electron is further away from the attractive force of the protons in the nucleus and is barely held in place. Lithium is very reactive and will readily share its outer electron.
Step-04:
The lithium-ion battery is composed of two electrodes – the cathode and the anode. The cathode is made of lithium nickel oxide and the anode is made of graphite. When the battery is connected to a charger, the lithium ions are liberated from the lithium nickel oxide crystal structure to the electrolyte solution. At the same moment, electrons are also liberated from the cathode and conducted to the anode.
Step-05:
The electrolyte solution is made of solvents and contains an additive (such as vinylene carbonate). It also contains a salt of lithium which, when dissolved in a solvent, separates into positive and negative ions with solvation shells. This creates a soup of positive and negative ions which always tries to maintain a neutral charge.
Step-06:
When an electron is conducted from the cathode, it bonds with the lithium ions from the electrolyte solution and forms a protective film on the graphite particles at the anode. This layer is called a solid electrolyte interphase (SEI). The lithium ions will now pass through this layer to enter the particles.
Step-07:
As the battery is charged, the lithium ions drift over from the cathode side and are absorbed by the anode. The electrons that are conducted to the anode pull positive lithium ions from the electrolyte solution, creating a concentration gradient. When the battery is mostly charged, some of the lithium ions from the cathode are finally absorbed by the anode.
A lithium-ion battery works by releasing lithium ions from the cathode and electrons from the anode. The lithium ions drift over to the anode and the electrons are conducted to the anode. This creates a concentration gradient and a protective layer (SEI) is formed on the graphite particles at the anode. This process is repeated when the battery is discharged.
Charging and Discharging: The Lifecycle of a Battery
Battery cycles are an important consideration when it comes to deep-cycle batteries. One battery cycle involves discharging the battery to a certain point and then recharging it to full. This is referred to as the Depth of Discharge (DoD).
For example, a 100 Amp Hour battery that is discharged to 12.3V, would be considered at approximately 30% depth of discharge. Deep-cycle batteries are rated for a certain number of cycles at a given depth of discharge – this is known as cycle life.
A deep-cycle battery might be rated for 1600 cycles at 30% DoD, but may only achieve 800 cycles at 50% DoD.
It is important to note that discharging the battery 100% on occasion will reduce its life, so it is best to only discharge it to the necessary level before recharging.
Additionally, it is a good idea to give the battery a “freshening” charge every few months when it is being stored.
Final Words
In conclusion, batteries play a crucial role in our daily lives, powering various electronic devices and providing energy storage solutions for a wide range of applications. By understanding how a battery works and the form of energy that batteries store, we can better appreciate the science behind these essential components.
As we have seen, the flow of electricity from a battery relies on chemical reactions that generate electric current. This current then powers electronic devices through a circuit. The rechargeable batteries we commonly use today can be recharged multiple times, thanks to the reversible chemical reactions that occur during the charging process.
However, it is important to note that rechargeable batteries may not keep their charge indefinitely, and their performance may vary depending on factors such as temperature, usage patterns, and specific battery chemistry. To make the most of your rechargeable batteries, it’s helpful to know how long they last when in use.
Batteries come in various sizes and types, including the widely used AA batteries. The term “battery” has its origins in the early days of electricity, when multiple electrochemical cells were connected together to form a “battery” of cells. Learn more about why it’s called a battery on our website.
By incorporating this knowledge into our daily lives, we can make informed decisions about battery usage and contribute to a more sustainable future. Continued research and development in battery technology will undoubtedly lead to further advancements and improvements, enhancing the efficiency and longevity of the batteries we rely on every day.
FAQ
What is the main form of energy that batteries store?
Batteries store energy in the form of chemical energy. This chemical energy is converted into electrical energy when the battery is connected to a circuit, allowing the flow of electricity to power electronic devices.
What are the key components of a battery and their functions?
A battery typically consists of an anode (negative electrode), a cathode (positive electrode), an electrolyte, and a separator. The anode and cathode facilitate the chemical reactions that generate electric current, while the electrolyte allows the flow of ions between the anode and cathode. The separator keeps the anode and cathode apart, preventing short circuits.
What is the difference between primary and secondary batteries?
Primary batteries are non-rechargeable, meaning they can only be used once before being discarded. Common examples include alkaline and zinc-carbon batteries. Secondary batteries, on the other hand, are rechargeable and can be used multiple times. Lithium-ion, nickel-metal hydride, and lead-acid batteries are examples of secondary batteries.
How do rechargeable batteries work, and how can they be recharged?
Rechargeable batteries work by utilizing reversible chemical reactions that convert chemical energy to electrical energy during discharging and restore the chemical energy during charging. To recharge a rechargeable battery, an external voltage is applied, reversing the chemical reactions and restoring the chemical energy stored within the battery.
What factors can affect the performance and life of a battery?
Several factors can impact a battery’s performance and lifespan, including temperature, usage patterns, and battery chemistry. High or low temperatures can reduce battery efficiency, while frequent charging and discharging can shorten the battery’s life. Different battery chemistries have varying capacities, charge/discharge rates, and lifespans, which also affect overall performance.
