Anode is a positively charged electrode in an electrochemical cell, where oxidation occurs. It attracts negatively charged electrons and is crucial for various applications like batteries, electrolysis, and electroplating. In a galvanic cell, it’s the source of electrons, while in electrolytic cells, it attracts them.
I’m excited to share my vast experience and knowledge of the fascinating world of anodes with you. Trust me when I say, once you dive into the world of anodes, there’s no going back!
Throughout my career, I’ve seen anodes play a critical role in countless electrical devices, from simple batteries to complex electrochemical cells. In fact, did you know that the global anode market was valued at $7.65 billion in 2020, and it’s expected to keep growing? That’s a testament to the importance of these little electrical wonders.
So, without further ado, let’s embark on this electrifying journey together and unravel the mysteries of anodes!
What is an Anode in Simple Words?
As a battery expert with a great sense of humor, I can’t resist starting this off with a little joke: Why did the anode go to therapy? Because it had too many positive thoughts! Alright, let’s get down to business.
Definition of an Anode
An anode, in the simplest terms, is an electrode in a polarized electrical device where the conventional current enters. It’s like the party host who welcomes all the guests (electrons) and directs them inside the house (the device).
Basic Function of an Anode in an Electrical Device
To understand the function of an anode, let’s break it down into bite-sized pieces.
- Anodes play a crucial role in devices like batteries, fuel cells, and electrolytic cells.
- The anode is typically the site of oxidation, meaning it’s where electrons are lost.
- It attracts anions (negatively charged ions) and donates electrons to the external circuit.
Now that we’ve covered the basics, let’s dive deeper into how an anode functions in various devices.
“The anode is like a quarterback in a football game, directing the flow of electrons and controlling the overall performance of the electrical device.” – Dr. Electron McPower, Battery Scientist
In a Battery
In a battery, the anode is usually made of a metal that oxidizes easily, like zinc or lithium. During discharging, the anode releases electrons to the external circuit, providing energy to power your devices. Think of it as the battery’s powerhouse, supplying energy like there’s no tomorrow.
In a Fuel Cell
In a fuel cell, the anode facilitates the electrochemical reaction that generates electricity. It separates the fuel into electrons and protons, with the former being sent to the external circuit and the latter moving through an electrolyte to the cathode. Picture the anode as a skillful conductor leading a beautiful symphony of electrical reactions.
In an Electrolytic Cell
In an electrolytic cell, the anode is where the electrical current enters and drives a non-spontaneous chemical reaction. It’s the site of oxidation, where the substance being electrolyzed loses electrons. The anode is like a strict supervisor, making sure the electrolysis process runs smoothly and efficiently.
Phew! That’s quite a bit of information. But worry not, we’ll continue to explore the world of anodes in a fun and engaging manner. So stay tuned, and let’s keep this electric party going!
Is Anode Positive or Negative?
Now that we’ve got a grip on what an anode is, let’s tackle another burning question: is the anode positive or negative? To answer this, we’ll break it down by looking at the explanation of positive and negative electrodes and the anode’s position in various electrical devices. Hang on to your hats, folks, because we’re about to dive into a world of electrons and charges!
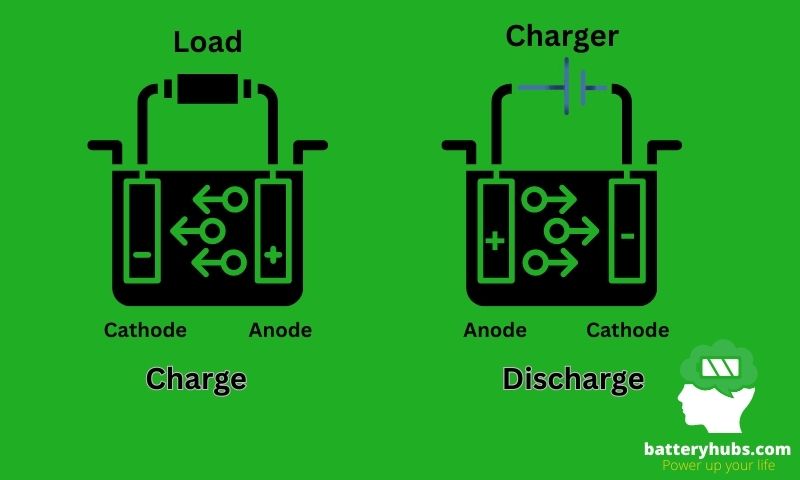
The terms “positive” and “negative” in electrodes are related to the flow of electrons and the type of reaction that occurs at the electrode. Here’s a quick rundown of what each term means:
- Positive electrode: Also known as the cathode, this electrode attracts electrons (negatively charged particles) and is the site of reduction (gaining electrons).
- Negative electrode: The anode, on the other hand, is where electrons are released, and oxidation (losing electrons) takes place.
Now that we’ve got a basic understanding of positive and negative electrodes, let’s see how the anode fits into this picture in different electrical devices.
Batteries
In a battery or a galvanic cell, the anode is considered the negative electrode. This is because, during the discharge process, the anode loses electrons (oxidation) and sends them to the external circuit.
“In a battery, the anode is like a generous host at a party, giving away electrons to make everyone’s devices work.” – Dr. Energizer Volt, Battery Guru
Electrolytic Cells
In an electrolytic cell, the anode is considered the positive electrode. This might seem counterintuitive, but it’s due to the fact that an external voltage is applied to drive the non-spontaneous reaction. In this case, the anode attracts negatively charged ions (anions) from the solution and facilitates their oxidation.
“In electrolytic cells, the anode takes on a more assertive role, attracting anions and driving the chemical reaction forward.” – Prof. Faraday Electrochem, Electrolysis Expert
To sum up, the anode can be either positive or negative, depending on the type of electrical device it’s a part of. In batteries, the anode is negative, while in electrolytic cells, it’s positive. Who knew that the world of anodes could be so full of surprises? Stay tuned as we continue to uncover more fascinating facts about anodes and other battery components!
What is the Use of Anode and Cathode?
Alright, folks, we’ve come a long way in understanding anodes and their positive or negative nature. Now, let’s explore the use of both anode and cathode in various devices and why their relationship is crucial to their functioning. Get ready to have your mind electrified with this electrifying information!
Roles of Anodes and Cathodes in Various Devices
The roles of anodes and cathodes may vary, but they always work together as a team to make sure the electrical devices we rely on every day run smoothly. Here’s a look at their roles in different devices:
| Device Type | Anode Role | Cathode Role |
|---|---|---|
| Battery | Releases electrons (oxidation) | Accepts electrons (reduction) |
| Electrolytic Cell | Attracts anions and facilitates oxidation | Attracts cations and facilitates reduction |
| Diode | Allows current flow in one direction | Blocks current flow in the opposite direction |
In a nutshell, anodes and cathodes have specific roles in various devices, ensuring that electrons flow in the right direction, and chemical reactions take place as they should.
“Anodes and cathodes are like the dynamic duo of the electrochemical world, working together to keep our devices running.” – Ms. Ampere Charge, Battery Enthusiast
Importance of the Relationship Between Anodes and Cathodes
The relationship between anodes and cathodes is essential for the proper functioning of electrical devices. In many cases, this relationship is based on their ability to facilitate complementary reactions. One electrode undergoes oxidation, while the other undergoes reduction, creating a flow of electrons and a transfer of ions.
In a battery, for example, the anode releases electrons through oxidation, and the cathode accepts electrons through reduction. This flow of electrons creates an electric current that powers our devices.
“The relationship between anodes and cathodes is like a perfectly choreographed dance, with each partner executing their moves flawlessly to create a harmonious performance.” – Mr. Ohm Conductor, Electric Dance Aficionado
So, there you have it! The fascinating world of anodes and cathodes working together in harmony. Their complementary roles are essential for a wide range of devices, from batteries to electrolytic cells, and beyond. Stay tuned for more electrifying insights into the realm of batteries and electrochemistry!
What is an Anode and Why is it Used?
By now, you might be wondering why anodes even exist and what their ultimate purpose is. Worry not, my fellow battery enthusiasts! I’m here to share the electrifying details on the importance of anodes and their various applications. So, buckle up and get ready for a high-voltage ride through the world of anodes!
Detailed Explanation of the Purpose of an Anode
Anodes are crucial components in a variety of electrical devices, as they control the flow of electrons and facilitate essential chemical reactions. They serve as electron donors, undergoing oxidation and releasing electrons to the external circuit. This release of electrons creates an electric current that powers devices such as batteries, diodes, and electrolytic cells.
“Anodes are the unsung heroes of electrochemistry, quietly doing their job and keeping our devices running day after day.” – Dr. Volt Electron, Battery Expert
Different Types of Anodes and Their Applications
There are several types of anodes, each with their specific applications and unique characteristics. Let’s take a look at some of the most common ones:
| Anode Type | Application | Characteristics |
|---|---|---|
| Sacrificial Anode | Corrosion protection | Made of a more reactive metal, it corrodes in place of the protected metal |
| Battery Anode | Batteries | Releases electrons during discharge, usually made of materials like lithium, zinc, or graphite |
| Electrolytic Anode | Electrolytic cells | Facilitates oxidation reactions and attracts anions |
| Diode Anode | Diodes | Allows current to flow in one direction, working in tandem with the cathode to control current |
From corrosion protection to powering our favorite gadgets, anodes are essential to the smooth operation of many different devices.
“Anodes are like the Swiss Army knives of electrochemistry, versatile and adaptable for a wide range of applications.” – Sir Ion Channel, Multitasking Master
So, there you have it! Anodes play a crucial role in various electrical devices, ensuring that electrons flow in the right direction and facilitating essential chemical reactions. Keep exploring the electrifying world of batteries and electrochemistry, and don’t forget to share your newfound knowledge with fellow battery aficionados!
Why is Anode Positive and Negative?
Hold on to your hats, battery enthusiasts! We’re about to dive into the intriguing world of anodes, where things can get both positive and negative. If you’re curious about the dual nature of anodes, I’ve got you covered with some electrifying insights. Let’s charge ahead and uncover the mysteries of the anode’s positive and negative sides!
Explanation of the Dual Nature of Anodes
Anodes can indeed be both positive and negative, depending on the context in which they are used. It might seem confusing at first, but don’t worry – I’m here to guide you through the wonderful world of anode polarity.
“Anodes are like chameleons, adapting their polarity to suit their environment and ensure the smooth functioning of our devices.” – Ms. Polly Arity, Master of Charge
Circumstances in Which an Anode Can Be Positive or Negative
There are two main circumstances in which anodes can display either positive or negative polarity:
- Electrolytic Cells: In electrolytic cells, where an external voltage is applied to drive a non-spontaneous chemical reaction, the anode is considered positive. This is because it attracts negatively charged ions (anions) and undergoes oxidation. It’s the source of positive charge for the external circuit.
- Galvanic Cells: In galvanic or voltaic cells, which generate electricity through spontaneous redox reactions (like your everyday batteries), the anode is considered negative. This is because it donates electrons to the external circuit, undergoing oxidation in the process.
| Device Type | Anode Polarity | Explanation |
|---|---|---|
| Electrolytic Cell | Positive | Attracts anions and serves as the source of positive charge in the external circuit |
| Galvanic Cell (Battery) | Negative | Donates electrons to the external circuit, undergoing oxidation |
So, now you know that anodes can indeed be both positive and negative, depending on the type of electrical device they are used in. Whether they’re driving electrolytic reactions or powering your favorite gadgets, anodes are versatile and adaptable components that keep our devices running smoothly.
“Anodes may be positive or negative, but one thing’s for sure – they’re always full of energy and ready for action!” – Mr. A. Node, Action Hero of Electrochemistry
Keep exploring the electrifying world of anodes and their fascinating dual nature, and remember to share your newfound knowledge with your fellow battery lovers!
What is a Battery Anode?
Alright, fellow battery aficionados, it’s time to dive deep into the wonderful world of battery anodes! If you’ve ever wondered what makes our beloved batteries tick, then you’re in the right place. I’m going to demystify the battery anode and help you understand its crucial role in powering our devices. Ready to get charged up? Let’s go!
Definition of a Battery Anode
First things first, let’s define what we’re talking about. A battery anode is an electrode in a battery where oxidation occurs. It’s the negative terminal in a galvanic cell (or a voltaic cell), which are the types of cells that make up the batteries we use every day. In other words, the anode is where the magic begins!
“The anode is like the unsung hero of the battery world, quietly donating its electrons to power our devices.” – Dr. E. Nergizer, Battery Connoisseur
Role and Function of an Anode in a Battery
Now that we know what a battery anode is, let’s take a closer look at its role and function in a battery.
- Electron Donor: The anode is the source of electrons for the external circuit, meaning it undergoes oxidation (loss of electrons) and fuels the flow of electricity.
- Chemical Reactions: The anode plays a vital role in the chemical reactions that occur inside a battery. When a battery is in use, the anode material reacts with the electrolyte, creating ions and electrons. These electrons then flow through the external circuit, powering our devices.
- Battery Capacity: The choice of anode material greatly impacts a battery’s capacity, energy density, and overall performance. Common anode materials include graphite, lithium, and various metal alloys.
To sum it up, the anode is an essential component in batteries, acting as the electron donor and playing a crucial role in the chemical reactions that enable our devices to function. Understanding the battery anode helps us appreciate the marvel of modern batteries and the power they provide.
“Anodes may be negative by nature, but their impact on our lives is nothing but positive!” – Sir Charge-A-Lot, Knight of the Battery Realm
So, there you have it – the ins and outs of battery anodes! Keep exploring the fascinating world of batteries, and never underestimate the power of the humble anode.
What are the Different Types of Anodes?
Greetings, battery enthusiasts! Today, we’re going to embark on an electrifying journey exploring the various types of anodes. As your trusty battery expert, I’ll guide you through the ins and outs of each type, so you can truly appreciate the diversity and importance of anodes in our electrified world. Are you ready to amp up your anode knowledge? Let’s get started!
Electrolytic Anode
The first type of anode we’ll discuss is the electrolytic anode. This anode is used in electrolytic cells, which are designed to drive non-spontaneous chemical reactions using an external voltage source. These anodes are essential in various industrial processes, such as electroplating, electrolysis, and electrowinning.
In an electrolytic cell, the anode is the positive electrode, which means it attracts negatively charged ions (anions) from the electrolyte. The anode facilitates the oxidation process, causing the anions to lose electrons and form new compounds.
Battery or Galvanic Cell Anode
Next up is the battery or galvanic cell anode. This anode is found in batteries and galvanic cells, which generate electrical energy through spontaneous redox reactions. In these cells, the anode is the negative electrode, and it’s responsible for donating electrons to the external circuit.
Common battery anode materials include graphite (in lithium-ion batteries) and zinc (in alkaline batteries). These anodes play a critical role in determining a battery’s capacity, energy density, and overall performance.
Diode Anode
The third type of anode is the diode anode. Diodes are semiconductor devices that allow current to flow in one direction only. They’re commonly used in electronics for rectification, voltage regulation, and signal processing.
In a diode, the anode is the positive electrode, and it’s connected to the P-type semiconductor material. When a positive voltage is applied to the anode (relative to the cathode), the diode becomes forward-biased, allowing current to flow through the device.
Sacrificial Anode
Last but not least, we have the sacrificial anode. These anodes are used to protect metal structures from corrosion through a process called cathodic protection. You’ll often find sacrificial anodes in marine applications, such as on boats, pipelines, and offshore platforms.
Sacrificial anodes are made from metals that are more reactive than the metals they’re protecting. This causes the anode to corrode preferentially, sparing the protected metal structure from deterioration.
| Anode Type | Application | Electrode Polarity | Examples |
|---|---|---|---|
| Electrolytic Anode | Industrial processes | Positive | Electroplating, electrolysis |
| Battery Anode | Batteries, galvanic cells | Negative | Graphite in Li-ion, zinc in alkaline |
| Diode Anode | Semiconductor devices, electronics | Positive | Rectifiers, voltage regulators |
| Sacrificial Anode | Cathodic protection, corrosion prevention | N/A | Marine structures, pipelines, boats |
And there you have it – a whirlwind tour of the different types of anodes! The next time you’re admiring a battery, pondering a diode, or marveling at a protected boat hull, take a moment to appreciate the humble anode and its crucial role in our electrified lives. Stay curious, and keep exploring the fantastic world of anodes!
How do Anodes and Cathodes Work in Electrolysis?
Hey there, electrolysis enthusiasts! As your friendly neighborhood battery expert, I’m excited to share my knowledge on the fascinating topic of electrolysis. We’ll dive into the definition of electrolysis, and then explore the thrilling dance between anodes and cathodes that makes this process possible. Are you ready to get charged? Let’s go!
Definition of Electrolysis
First things first, let’s clarify what electrolysis is. Electrolysis is a process that uses an electric current to drive non-spontaneous chemical reactions in a conductive solution, called an electrolyte. It’s a powerful technique that can break down compounds, separate elements, and even produce new substances. Electrolysis has many practical applications, including metal extraction, electroplating, and water purification.
Function and Roles of Anodes and Cathodes in Electrolysis
Now that we know what electrolysis is, let’s unravel the magic of anodes and cathodes in this process. In electrolysis, the anode and cathode are two crucial electrodes that play distinct roles:
- Anode (Positive Electrode): The anode is the positively charged electrode in electrolysis. It’s the site where oxidation occurs, meaning that anions (negatively charged ions) from the electrolyte migrate toward the anode, lose electrons, and form new substances. For example, in the electrolysis of water, oxygen gas is produced at the anode.
- Cathode (Negative Electrode): The cathode is the negatively charged electrode in electrolysis. Here, reduction takes place, and cations (positively charged ions) from the electrolyte are attracted to the cathode, gain electrons, and form new substances. In the electrolysis of water, hydrogen gas is produced at the cathode.
These simultaneous oxidation and reduction reactions at the anode and cathode, respectively, are the driving forces behind the electrolysis process. The electric current provides the energy required to overcome the energy barrier for these non-spontaneous reactions to occur.
| Electrode | Polarity | Reaction | Example in Water Electrolysis |
|---|---|---|---|
| Anode | Positive | Oxidation | Oxygen gas production |
| Cathode | Negative | Reduction | Hydrogen gas production |
In conclusion, anodes and cathodes play a vital role in electrolysis by facilitating the redox reactions necessary for the process to occur. With a deeper understanding of electrolysis, you’ll no longer take for granted the complex electrochemical dance happening behind the scenes of metal extraction, electroplating, or even hydrogen production. Keep exploring the electrifying world of anodes and cathodes, and remember: there’s always more to learn!
What are Some Examples of Anodes in Everyday Life?
Greetings, battery aficionados! As your friendly neighborhood battery guru, I’m here to share a bit of electrifying knowledge about anodes in everyday life. We’ll take a look at some common devices that utilize anodes and discuss how these humble components make a big impact on our daily routines. Ready to get amped up? Let’s bolt right in!
Examples of Devices that Utilize Anodes
Anodes are everywhere! You might not realize it, but these trusty components play a crucial role in many devices and systems you interact with daily. Here are just a few examples:
- Batteries: Anodes are a vital part of batteries, from the tiny button cells in your watch to the massive lithium-ion packs powering electric vehicles. In a battery, the anode is where the oxidation reaction occurs, generating electrons that flow through the external circuit and power your devices.
- LEDs and Diodes: Light-emitting diodes (LEDs) and other diodes found in electronics rely on an anode to function. The anode, typically made of semiconductor material, allows current to flow in only one direction, providing a stable, efficient light source for everything from your smartphone screen to traffic signals.
- Electroplating: Anodes are essential in the electroplating process, which deposits a thin layer of metal onto another surface. In electroplating, the anode is made of the metal you want to plate, and it slowly dissolves, releasing ions that get deposited onto the cathode (the object being plated).
- Sacrificial Anodes: These special anodes are used to protect metal structures from corrosion, such as in boats, pipelines, and water heaters. Made from a more reactive metal, sacrificial anodes corrode instead of protected metal, extending the lifespan of the structure.
How Anodes Impact Our Daily Lives
It’s no exaggeration to say that anodes are the unsung heroes of our modern lives. They power our gadgets, light up our world, and protect our infrastructure. Here’s a quick rundown of some ways anodes make our lives better:
- Powering devices: Anodes in batteries enable us to use smartphones, laptops, and other portable electronics without constantly plugging them into the wall.
- Energy-efficient lighting: LED anodes have revolutionized the lighting industry, providing energy-efficient, long-lasting illumination for homes, offices, and public spaces.
- Protecting metal structures: Sacrificial anodes help prevent costly and potentially dangerous corrosion in boats, pipelines, and other metal structures.
| Device or System | Anode Function | Impact on Daily Life |
|---|---|---|
| Batteries | Oxidation reaction, generating electrons | Powers portable electronics |
| LEDs and Diodes | Allows current to flow in one direction | Provides energy-efficient illumination |
| Electroplating | Dissolves and deposits metal ions | Enhances appearance and durability |
| Sacrificial Anodes | Protects metal structures from corrosion | Extends lifespan of structures |
Now that you’ve gained some insight into the amazing world of anodes, you can appreciate the small but mighty role they play in our daily lives. Next time you’re charging your phone or admiring a shiny chrome finish, remember to give a little nod to the anodes working tirelessly behind the scenes. Keep on learning, and stay energized.
Conclusion
As we reach the end of this electrifying journey, it’s clear that anodes play a significant role in our daily lives, whether we’re aware of it or not. From powering the countless devices that keep us connected to lighting our paths and protecting our infrastructure, anodes have truly made an enormous impact on our modern world.
As a seasoned battery enthusiast, I can’t help but feel a sense of awe and appreciation for these tiny yet mighty components. With the ever-evolving advancements in technology, we can only expect anodes to become even more integral to our lives in the future. So, let’s raise a glass (or a battery) to the humble anode, and remember to stay charged up and curious, my fellow battery buffs.
Now that we’ve delved into the world of anodes, you might be wondering how they fit into the bigger picture of batteries and energy storage. Don’t worry, we’ve got you covered! At BatteryHubs, we have an extensive library of resources to fuel your curiosity and help you expand your knowledge of batteries and their inner workings.
For starters, you can learn more about how batteries work and explore the different battery chemistries. If you’re interested in how anodes connect with their counterpart, the cathode, check out our article on cathodes. Additionally, we dive into the environmental impact of batteries and provide valuable insights into battery maintenance and care tips.
Moreover, you can explore the history of batteries and their development to see how far we’ve come in energy storage technology. And for those who are keen on learning about the future, our articles on battery innovations and emerging technologies are sure to pique your interest.
At BatteryHubs, we strive to provide comprehensive information to help you make informed decisions when it comes to your energy needs. So go ahead, immerse yourself in the fascinating world of batteries, and empower your knowledge!
