A battery is an electrochemical device that converts chemical energy into electrical energy. It consists of one or more electrochemical cells, each containing a cathode, an anode, and an electrolyte. Batteries store and release energy through redox reactions, allowing for portable power in various applications, such as electronic devices and electric vehicles.
It’s me, your friendly battery expert with oodles of experience in this electrifying world. I know, I know, you’re probably thinking, “What is a battery, and why should I care?” Well, let me tell you, batteries are the unsung heroes of modern life, powering everything from our smartphones to electric cars. But what is a battery? It’s a magical (not really, but kinda) little device that stores and releases electrical energy, transforming our lives in countless ways. I’ve been in this game for years, and I can’t help but chuckle at how these tiny powerhouses have taken over the world. So, sit back, relax, and let’s dive into the fascinating world of batteries together!
Ready to dive deeper into the science behind batteries? Let’s go! We’ll start with a closer look at the electrochemical reactions happening in these marvelous devices, and then explore their key components. Trust me, you’re in for a treat!
Why it is called a battery?
The term “battery” has its origins in the 18th century, and its usage in the context of energy storage can be traced back to the experiments of Benjamin Franklin. Initially, the word “battery” referred to a group of similar objects that were used collectively to perform a specific function. In the field of artillery, a battery referred to a series of cannons arranged in a row to produce a powerful effect when used together.
In 1749, Benjamin Franklin conducted electricity experiments using a series of connected capacitor elements. He recognized a parallel between this arrangement and the artillery concept of a battery, in that both produced a greater overall effect when their components were combined. The term “battery” was thus applied to the series of capacitor elements, symbolizing the amplification of their electrical effect, much like the increased firepower of cannons when arranged in a battery formation.
Since that time, the word “battery” has been used to describe any configuration of multiple energy sources connected together to achieve a more potent outcome. This applies to various energy storage devices, from common AAA batteries to large arrays of solar panels on rooftops.
The continued use of the term “battery” highlights the power of language and the ability of a single word to encapsulate a complex concept. The enduring nature of this term serves as a reminder of the history behind it, as well as the ingenuity of Benjamin Franklin in drawing an analogy between artillery batteries and electrical energy storage configurations.
The Science Behind Batteries
Batteries, my friends, are like little chemistry labs, converting chemical energy into electrical energy through a series of reactions. Electrochemical cells, the building blocks of batteries, are what make this magic happen. They’re like the superheroes of the battery world!
Key components of a battery
Every superhero needs a sidekick, and for electrochemical cells, that means electrodes and electrolytes. Let’s break it down:
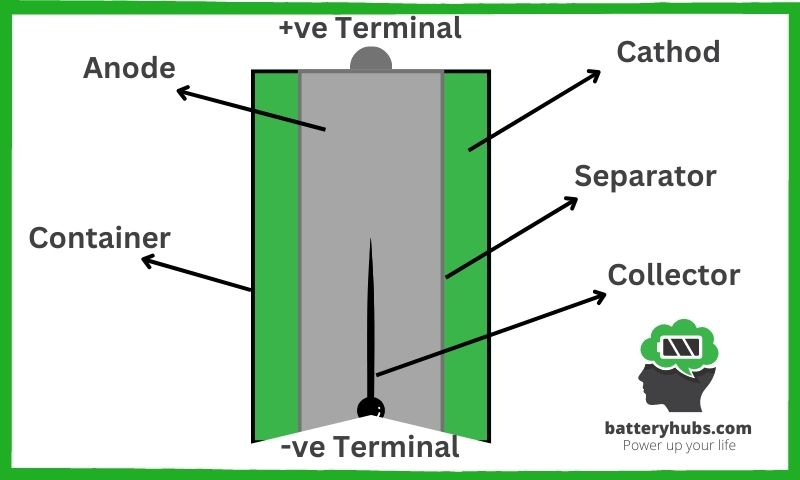
- Electrodes: Anode (negative) and cathode (positive)
These two opposing forces are essential for battery life. When a battery is connected to a circuit, the anode undergoes an oxidation reaction, losing electrons:
Anode(-) → Anode(+) + e-
The cathode, on the other hand, gains electrons through a reduction reaction:
Cathode(+) + e- → Cathode(-)
- Electrolyte: The medium that enables ion flow
The electrolyte is the referee of this electron tug-of-war, facilitating the flow of ions between the anode and cathode. When the battery is in use, the anode releases positively charged ions into the electrolyte, which then move toward the cathode, creating an electric current in the external circuit.
The working mechanism of batteries
Imagine you have a group of tiny people called “electrons” who live in a battery-powered toy. The toy has two homes: the “Anode House” and the “Cathode House.” When you turn on the toy, the electrons want to go from the Anode House to the Cathode House. To do that, they need to walk through a special path called a “circuit.”
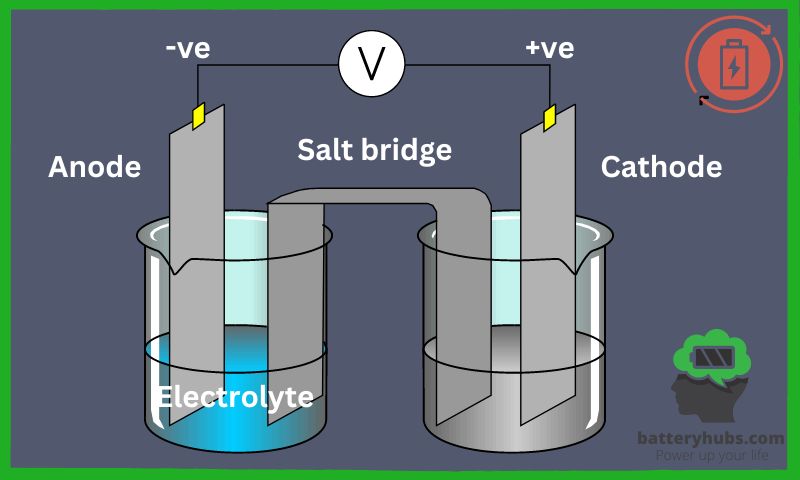
There’s a bridge between the two houses called the “Electrolyte Bridge.” The electrons can’t walk on this bridge, but they send their tiny friends, called “ions,” across it. When the ions reach the other side, the electrons can finally move to the Cathode House, and your toy starts to work.
When your toy runs out of energy, you recharge the battery, and the electrons go back to the Anode House, ready for another fun day of playing with your favorite toy.
Now that we’ve taken a playful detour to explain batteries, let’s return to our more technical adventure. We’ll dive back into electrochemical reactions and charge-discharge cycles, which are essential for understanding how batteries power our devices.
- Electrochemical reactions
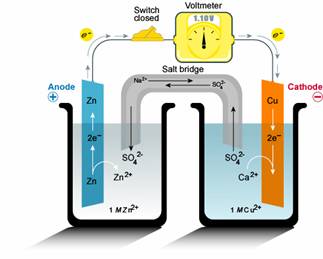
The overall redox (reduction-oxidation) reaction in a battery is the result of these individual anode and cathode reactions. In simple terms, the anode loses electrons, and the cathode gains them. The magic lies in the electrolyte, which keeps the whole thing going.
- Charge and discharge cycles
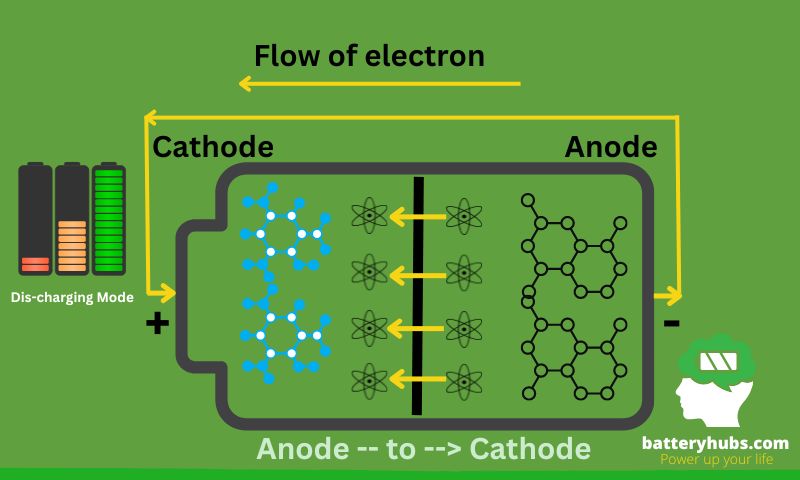
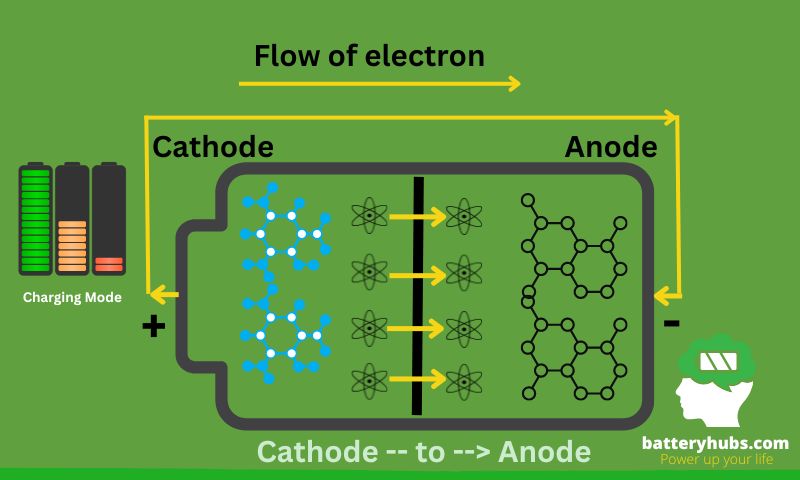
Batteries, especially rechargeable batteries, go through cycles of charging and discharging. Charging restores the chemical energy, while discharging converts it into electrical energy for use in devices.
Imagine you’re with a toy car powered by a rechargeable battery. When the car runs out of energy, you plug it in, and the battery charges. The charging process replenishes the battery’s energy capacity, so you can unplug it and play with the car again. The cycle continues as long as the battery remains functional.
I hope you’re enjoying this electrifying adventure! Stay tuned for more fascinating insights into the world of batteries, including different types, performance factors, and safety concerns.
Now that we’ve covered the science behind batteries, let’s dive into the different types of batteries out there. Buckle up, because we’re about to explore the fascinating world of primary batteries, which are the single-use heroes of our everyday lives!
Exploring Different Types of Batteries
There are two main types of batteries:
- Primary
- Secondary
1. Primary batteries (non-rechargeable)
Primary batteries are single-use and cannot be recharged, while secondary batteries are rechargeable. Within these two categories, there are many different types of batteries, each with their own advantages and disadvantages. Primary batteries include Carbon Zinc, Alkaline, Lithium, Silver Oxide, and Zinc Air batteries.
These one-and-done wonders have a special place in my heart. They’re the batteries that keep our remote controls, toys, and flashlights going. Let’s take a closer look at some common primary battery types:
- Alkaline batteries
Alkaline batteries are the workhorses of the disposable battery world. They’re used in a wide range of electrical devices thanks to their affordable price, long shelf-life, and ability to deliver steady power. In fact, they’re the go-to choice for most people when they need a quick battery fix!
- Zinc-carbon batteries
Zinc-carbon batteries, also known as dry cells, are the granddaddies of primary batteries. These old-timers have been around since the days of Benjamin Franklin and his brass hooks. Although not as popular as alkaline batteries due to their lower energy density and shorter shelf-life, they still have their place in low-drain devices like wall clocks.
- Lithium batteries
Ah, lithium batteries – the high-energy, long-lasting superstars of the primary battery world. These bad boys are used in devices that demand a lot of juice, like digital cameras and high-performance flashlights. Their lightweight design and ability to withstand extreme temperatures make them ideal for a variety of portable devices.
2. Secondary batteries (rechargeable)
Secondary batteries include Rechargeable Alkaline, Nickel-Cadmium, Nickel-Metal Hydride, Lithium-Ion, and Lead-Acid batteries. Choosing the best battery for your needs depends on your particular application and the performance requirements, so it is important to research the different types of batteries available.
Just when you thought we’ve seen it all, get ready for the fantastic world of secondary batteries! These rechargeable powerhouses keep our gadgets alive and kicking. So, without further ado, let’s delve into some of the most popular rechargeable battery types!
- Lead-acid batteries
If you’ve ever owned a car, you’ve likely encountered a lead-acid battery. These heavyweight champions are the go-to choice for starting, lighting, and ignition (SLI) applications. They’re also great at providing high electric current for short periods of time, making them perfect for emergency power supplies and uninterruptible power systems (UPS).
- Nickel-cadmium (NiCd) batteries
NiCd batteries have a long and storied history, dating back to the days of the French physicist who invented them. They’re tough, reliable, and can withstand a wide range of temperatures. However, they’ve fallen out of favor due to their lower energy density and the bodily harm caused by their toxic cadmium content.
- Nickel-metal hydride (NiMH) batteries
The NiMH battery is like the cool younger sibling of the NiCd battery. It offers higher energy capacity and energy density, making it a popular choice for high-drain electronic devices like digital cameras, laptops, and cordless power tools. Plus, they’re more eco-friendly than their NiCd counterparts, which is a big win in my book!
- Lithium-ion (Li-ion) batteries
Li-ion batteries are the poster children of the rechargeable battery world. They’re in everything from smartphones to electric vehicles, and for good reason. They pack a lot of electric energy into a lightweight package, boast a long life, and can handle high voltages and currents. Just remember to handle them with care, as they can be sensitive to overcharging and overheating.
- Lithium-polymer (LiPo) batteries
LiPo batteries are the sleek and stylish cousins of Li-ion batteries. They use a liquid electrolyte in a flexible polymer casing, which allows them to be shaped into ultra-thin and lightweight formats. You’ll find them in high-end smartphones, tablets, and even drones. But beware – they can be a bit more finicky than their Li-ion relatives when it comes to charging and discharging.
Comparing battery types
Now that we’ve explored the magnificent world of battery types, it’s time for the ultimate showdown! Let’s compare these energizing wonders based on their energy density, charging and discharging features, and applications. Buckle up, folks – things are about to get electrifying!
- Energy density
Energy density is a measure of how much energy a battery can store per unit of volume or weight. In this category, lithium-ion and lithium-polymer batteries reign supreme, followed by NiMH batteries. Lead-acid and NiCd batteries bring up the rear with lower energy densities, but they still have their uses, as we’ll see later.
- Charging and discharging features
When it comes to charging and discharging, lithium-ion and lithium-polymer batteries take the cake for efficiency and speed. However, they can be sensitive to overcharging and overheating, so you’ll want to treat them with care. NiMH batteries are more robust, but they can suffer from the dreaded “memory effect,” which can reduce their overall capacity. Lead-acid and NiCd batteries can handle heavy loads and extreme temperatures but may require more frequent maintenance.
- Applications and use cases
Each battery type has its unique strengths and ideal applications:
- Alkaline and zinc-carbon primary batteries are perfect for low-drain devices like remote controls, flashlights, and smoke detectors.
- Lead-acid batteries excel in automotive applications, emergency power supplies, and UPS systems.
- NiCd batteries may be old-school, but they’re still used in power tools, aviation, and emergency lighting.
- NiMH batteries are great for high-drain devices like digital cameras, laptops, and cordless power tools.
- Lithium-ion and lithium-polymer batteries are the darlings of modern electronics, from smartphones and laptops to electric vehicles and drones.
| Battery Type | Energy Density | Charging & Discharging Features | Applications & Use Cases |
|---|---|---|---|
| Alkaline | Low | Slow charging, limited discharge cycles | Low-drain devices (remote controls, flashlights, smoke detectors) |
| Zinc-carbon | Low | Slow charging, limited discharge cycles | Low-drain devices (toys, radios) |
| Lead-acid | Moderate | Slow charging, deep discharge cycles, requires maintenance | Automotive applications, emergency power supplies, UPS systems |
| Nickel-cadmium (NiCd) | Moderate | Moderate charging, memory effect, requires maintenance | Power tools, aviation, emergency lighting |
| Nickel-metal hydride (NiMH) | High | Fast charging, memory effect, robust | High-drain devices (digital cameras, laptops, cordless power tools) |
| Lithium-ion (Li-ion) | Very High | Fast charging, sensitive to overcharging and overheating | Smartphones, laptops, electric vehicles, drones |
| Lithium-polymer (LiPo) | Very High | Fast charging, sensitive to overcharging and overheating | High-end electronics, smartphones, RC vehicles |
As we’ve explored the fascinating world of batteries, it’s time to dive into the factors that affect their performance. Trust me, understanding these factors will help you get the most out of your batteries, and you’ll feel like a battery guru!
Factors Affecting Battery Performance
Now, let’s talk about the nitty-gritty details of Factors Affecting Battery Performance.
A. Capacity
Battery capacity is measured in ampere-hours (Ah) or watt-hours (Wh). For instance, a 10 Ah battery can supply 10 amperes of current for 1 hour. To calculate watt-hours, multiply the Ah rating by the battery voltage (Wh = Ah x V). For example, a 10 Ah battery with 12V will have a capacity of 120 Wh (10 Ah x 12V).
Several factors influence battery capacity, such as:
- Battery Chemistry
- Size and weight
- Temperature
- Age and number of charge/discharge cycles
B. Discharge rate
The discharge rate affects battery life significantly. High discharge rates can lead to reduced capacity and shorter battery life. For example, lithium-ion batteries lose around 20% of their capacity after 1000 charge/discharge cycles at a 1C discharge rate (where C is the battery’s capacity).
To extend battery life, you can:
- Use devices with power-saving features
- Turn off unused functions on your devices
- Store batteries in a cool, dry place
C. Charging practices
Effective charging techniques are crucial for maintaining battery health. Some tips include:
- Avoid overcharging by using a charger with an automatic shut-off feature
- Charge your batteries before they are completely discharged
- Keep batteries at room temperature while charging
Let’s compare fast and slow charging in a tabular form:
| Charging Method | Pros | Cons |
|---|---|---|
| Fast Charging | Time-efficient, convenient | May cause more wear on the battery, generates heat |
| Slow Charging | Gentle on the battery, better longevity | Time-consuming, less convenient |
To ensure battery health, consider these factual data:
- A lithium-ion battery charged at 0.5C typically lasts for 1500-3000 cycles
- Charging at a 1C rate might reduce the cycle life to 500-1500 cycles
Now that we’ve mastered the factors affecting battery performance, let’s address another crucial aspect of batteries: safety and environmental impact. It’s essential to consider these factors as responsible battery users and guardians of our planet.
Battery Safety and Environmental Impact
So, let’s discuss Battery Safety and Environmental Impact.

A. Safety concerns and best practices
Batteries can pose some risks if not handled properly, such as overcharging and overheating. Some best practices to prevent these issues include:
- Using a charger with an automatic shut-off feature
- Avoiding exposure to direct sunlight or extreme temperatures
- Ensuring proper ventilation during charging
To prevent short circuits and reduce battery-related accidents, follow these tips:
- Keep batteries away from metal objects that could cause a short circuit
- Store batteries in a cool, dry place away from flammable materials
- Avoid puncturing or damaging the battery casing
Factual data indicates that adopting these safety measures can reduce battery-related accidents.
According to the U.S. Consumer Product Safety Commission, there were more than 25,000 battery-related emergency room visits between 1997 and 2010. By following these best practices, we can help decrease the number of accidents and make our electronic devices safer.
B. Environmental considerations
Batteries can have negative environmental impacts due to their hazardous components, such as lead, cadmium, and lithium. It’s essential to promote responsible battery disposal and recycling to minimize these impacts. Here’s a step-by-step guide to disposing of and recycling batteries:
- Identify your local battery recycling center or collection point.
- Collect your used batteries in a non-metal container to prevent short circuits.
- Transport the batteries to the recycling center or collection point, ensuring they are not exposed to extreme temperatures or direct sunlight.
Eco-friendly battery innovations are also on the rise, with researchers developing batteries made from organic materials and exploring new methods to extend battery life. By staying informed and adopting sustainable practices, we can help reduce the environmental impact of batteries while still enjoying the convenience they offer.
The Future of Batteries and Emerging Technologies
As we continue our electrifying journey into the world of batteries, let’s look toward the horizon and see what the future holds. In this section, we’ll delve into the cutting-edge developments that are set to revolutionize battery technology, from solid-state batteries to wireless charging.
A. Solid-state batteries: Benefits and potential applications
Solid-state batteries are the next big thing, folks! Replacing the liquid electrolyte with a solid one, these batteries offer numerous advantages, such as increased energy density, better safety, and a longer lifespan.
I’m already daydreaming about electric vehicles with extended driving ranges and smartphones that can last for days without recharging!
B. Sodium-ion batteries: A sustainable alternative
With lithium reserves becoming scarcer, sodium-ion batteries are stepping up as a sustainable alternative. Sodium, being abundant and low-cost, is a game-changer.
These batteries can store similar amounts of energy as lithium-ion batteries, making them perfect for large-scale energy storage applications.
Talk about a win-win situation for both our pockets and the environment!
C. Energy storage solutions for renewable energy sources
As the world shifts towards renewable energy, batteries play a crucial role in energy storage.
Technologies like flow batteries and pumped hydroelectric storage are being developed to support the integration of renewable sources into the grid.
I’m super excited about a future where renewable energy is efficiently harnessed and stored, leading to a cleaner and greener planet!
D. Wireless charging technologies: Revolutionizing battery charging
Imagine a world where you can charge your devices simply by placing them on a surface – no cords, no clutter.
Well, wireless charging technologies are making that dream a reality! Using electromagnetic fields, energy is transferred between two coils, one in the charging pad and the other in the device.
This technology is already being used for smartphones and electric toothbrushes, and I can’t wait to see it being adopted for other devices as well.
Conclusion
In this comprehensive guide, we’ve explored the fascinating world of batteries – from understanding how batteries work to the cutting-edge developments that are set to revolutionize the future. We’ve learned about the journey of electricity from a battery and the form of energy that batteries store. We’ve also delved into the role of batteries in circuits, and the intricacies of rechargeable batteries, including how they get recharged and how long they last.
We also discussed the ubiquitous AA batteries and the origins of the term battery. Throughout this journey, we’ve seen how batteries have evolved and the impact they’ve had on our daily lives. As an experienced battery enthusiast, I’m thrilled to witness the continuous innovations and developments in this field. And now, equipped with the knowledge and understanding of battery technology, you too can join me in appreciating the power that batteries bring to our lives. So, here’s to a bright and electrifying future, powered by batteries!
FAQ
What is the main difference between primary and secondary batteries?
Primary batteries are non-rechargeable, single-use batteries, while secondary batteries are rechargeable and can be used multiple times. Primary batteries, such as alkaline and zinc-carbon batteries, store energy through a chemical reaction that is not reversible. In contrast, secondary batteries, like lithium-ion and nickel-cadmium batteries, can have their chemical reactions reversed, allowing them to be recharged and reused.
How do batteries convert chemical energy into electrical energy?
Batteries use electrochemical reactions to convert chemical energy into electrical energy. Inside a battery, there are positive and negative electrodes and an electrolyte. The chemical reactions between the electrolyte and the electrodes generate a flow of electrons, creating an electric current that can power electronic devices.
What is energy density, and why is it important in batteries?
Energy density is the amount of energy stored per unit volume or weight of a battery. It is an important factor because it determines how much energy a battery can store and provide to a device. A higher energy density means that a battery can store more energy in a smaller and lighter package, which is particularly important for portable devices and electric vehicles.
How can I extend the life of my rechargeable batteries?
To extend the life of rechargeable batteries, follow these tips: (1) avoid overcharging and overheating, (2) use the appropriate charger for your battery type, (3) store batteries in a cool, dry place, and (4) avoid using your devices until the battery is completely drained.
How do solid-state batteries differ from traditional batteries?
Solid-state batteries use a solid electrolyte instead of a liquid or gel electrolyte found in traditional batteries. This design offers several benefits, including increased energy density, improved safety, and a longer lifespan. Solid-state batteries also have the potential for faster charging times and can be used in a variety of applications, such as electric vehicles and portable electronics.
Are there eco-friendly alternatives to traditional batteries?
Yes, there are eco-friendly alternatives to traditional batteries, such as sodium-ion batteries. Sodium-ion batteries use abundant and inexpensive sodium as the main component, making them a more sustainable option compared to lithium-ion batteries. Additionally, advancements in battery recycling and disposal practices help reduce the environmental impact of batteries.
Related Post:
