A cathode is the electrode in an electrical cell or vacuum tube where reduction occurs, and electrons are gained. In a cell, it’s the positive terminal, while in a vacuum tube, it’s the negatively charged component. It plays a crucial role in devices like batteries, diodes, and cathode ray tubes.
Cathodes play a pivotal role in the world of electrical devices, serving as a fundamental component in a wide range of technologies. As an essential element in various electronic systems, understanding the concept of a cathode not only helps enthusiasts and professionals alike to better grasp how these devices operate but also sheds light on the intricacies of electronic design.
In this section, I will explore the basics of cathodes and highlight their significance in the realm of electrical devices, setting the stage for a deeper understanding of their practical applications and the underlying science.
The Basic Definition of a Cathode
In general, a cathode is often referred to as the negative electrode in electrical devices, including diodes, vacuum tubes, and electrolytic cells. This critical component is responsible for allowing the flow of electrons into the device, thus enabling the current to travel through the circuit.
In this role, the cathode is responsible for facilitating electron transfer, which ultimately powers the electrical device.
The negatively charged nature of the cathode enables it to attract positive charges or cations from the surrounding environment, creating the necessary conditions for electric current to flow and ensuring proper device function.
While the cathode is typically thought of as the negative electrode in electrical devices, it can also function as the positive electrode in primary cells, such as batteries.
In this context, the cathode serves as the electron acceptor, receiving electrons from the external circuit during a redox (reduction-oxidation) reaction.
As electrons flow into the cathode, the positive charge of the electrode increases, allowing it to attract negatively charged particles or anions.
This process is crucial for the generation of electrical energy in primary cells, as it facilitates the transfer of electrons from the anode (negative electrode) to the cathode (positive electrode), thereby completing the circuit and powering the device.
Understanding this dual nature of cathodes is essential for grasping the complexities of various electrical systems and their applications.
Cathode and Anode: Understanding the Difference
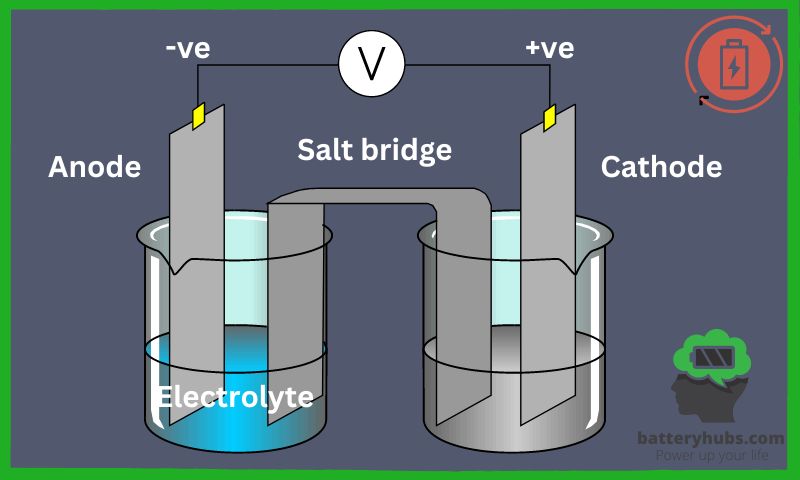
Alright, let’s dive into the world of anodes and cathodes! As a battery enthusiast with tons of experience, I can assure you that understanding the difference between anodes and cathodes is essential for mastering the battery game. So, what’s the deal with anodes?
Anodes, in contrast to cathodes, are the positive electrodes in most electrical devices, while cathodes are typically the negative electrodes. However, in primary cells (like batteries), the roles are reversed, with anodes being the negative electrodes and cathodes being the positive ones. Now, this might sound a bit confusing, but stick with me!
The primary difference between anodes and cathodes lies in the type of reactions they undergo during the electrochemical process. Anodes are involved in oxidation reactions, meaning they lose electrons, while cathodes participate in reduction reactions, where they gain electrons.
The Roles of Cathodes and Anodes in Electrical Devices
Now that we’ve got the basic definitions covered, let’s talk about how cathodes and anodes work together in electrical devices. Trust me, this is where the magic happens!
| Role | Cathode | Anode |
|---|---|---|
| Reaction | Reduction (gains electrons) | Oxidation (loses electrons) |
| Charge | Negative in most devices, positive in primary cells | Positive in most devices, negative in primary cells |
| Function | Attracts cations, allows electrons to flow into the device | Attracts anions, allows electrons to flow out of the device |
In electrical devices like batteries, cathodes and anodes work together to generate an electric current. As I mentioned earlier, anodes undergo oxidation reactions, losing electrons and creating a positive charge. These electrons are then attracted to the cathode, which undergoes reduction reactions and gains electrons, creating a negative charge. This flow of electrons from the anode to the cathode is what we call an electric current, which powers our devices.
In a nutshell, the dance between cathodes and anodes is the heart and soul of electrical devices. These two electrodes are the unsung heroes that keep our world electrified and our gadgets running smoothly. So, the next time you charge your phone or swap out your batteries, remember the incredible teamwork happening between cathodes and anodes, and give them a mental high-five!
Cathodes in Various Applications
As a battery enthusiast and someone who’s been around the block in the world of electronics, I’ve seen Cathodes do some pretty amazing things. They’re like the unsung heroes of our devices! Let’s explore the fascinating roles of cathodes in different applications, shall we?
A. Cathodes in Diodes
Diodes are nifty little components found in many electronic circuits. They’re like one-way streets for electrical current, allowing it to flow in only one direction. Cathodes play a crucial role in diodes by attracting the current, thus ensuring that it flows correctly.
In diodes, the cathode is usually marked with a band to help identify its orientation. When the cathode is more negative than the anode, the diode is in its “forward-biased” state, allowing current to flow. If the cathode is more positive, the diode is “reverse-biased,” and no current can pass through. This simple yet powerful characteristic of diodes makes them indispensable in various applications, from power conversion to signal processing!
B. Cathodes in Vacuum Tubes
Ah, vacuum tubes, the granddaddy of modern electronics! These devices played a massive role in the early days of electronics, and guess what? Cathodes were right there, front and center.
In vacuum tubes, cathodes are heated to release electrons through a process called “thermionic emission.” These electrons are then attracted to the positively charged anode, creating an electric current. Vacuum tubes were widely used in the 20th century for various applications, including amplification, rectification, and even early computers! Nowadays, they’re mostly used in specialized applications like guitar amplifiers, where they’re cherished for their warm, nostalgic sound.
C. Cathodes in Cathode Ray Tubes
Cathode ray tubes (CRTs) are another classic application of cathodes. In these devices, cathodes emit a beam of electrons that are accelerated and deflected by magnetic fields to strike a phosphor-coated screen. This process creates an image on the screen, which was the basis for early television and computer monitors.
Although CRT technology has largely been replaced by modern flat-panel displays, it’s still an excellent example of how cathodes have shaped our world. Plus, there’s something charming about the warm, fuzzy glow of a vintage CRT screen, don’t you think?
D. Cathodes in Oscilloscopes
Oscilloscopes are essential tools for visualizing electrical signals, and you guessed it, cathodes play a significant role here too. In fact, many oscilloscopes use a special type of CRT called an “electron beam” or “vector” CRT.
In these devices, the cathode emits a focused electron beam that traces the electrical signal on a phosphor-coated screen. This process allows engineers and technicians to view and analyze the waveform of the signal, helping them diagnose problems and optimize their circuits.
E. Cathodes in Batteries
Finally, let’s talk about my favorite application of cathodes: batteries! In batteries, cathodes are the positive electrodes where the reduction reactions take place. During discharge, the anode releases electrons that travel through the external circuit and are then accepted by the cathode. This flow of electrons powers our devices, and the magic of electrochemistry keeps the world running.
There are various types of batteries, each with its unique cathode materials, such as lithium cobalt oxide in lithium-ion batteries and lead dioxide in lead-acid batteries. These materials play a crucial role in determining the battery’s performance, capacity, and overall efficiency.
So, there you have it! Cathodes are incredibly versatile and essential components in a wide range of batteries, they’re truly the unsung heroes that keep our modern world humming along. It’s amazing how one little electrode can play such a significant role in so many different devices. As someone who’s been fascinated by batteries and electronics for years, I can’t help but appreciate the incredible impact cathodes have had on our lives.
Next time you power up your smartphone, watch an old TV show, or jam out on a vintage guitar amp, take a moment to appreciate the humble cathode. It’s been quietly shaping our world for over a century, and it’s still going strong. Who knows what exciting new applications we’ll discover for cathodes in the future? As an electronics enthusiast, I can’t wait to find out!
The Science Behind Cathodes
As someone who’s spent countless hours tinkering with batteries and electronics, I can’t help but be in awe of the science behind cathodes. It’s a fascinating world of oxidation, reduction, and electron flow that makes our favorite gadgets tick. Let me break it down for you in a way that’s both informative and fun.
Oxidation and Reduction Reactions
To understand the magic happening inside our batteries, we need to dive into the world of redox reactions. In a nutshell, oxidation is the process of losing electrons, while reduction is the process of gaining electrons. These two processes go hand in hand, and they’re essential for the proper functioning of a cathode.
* **Oxidation:** Loss of electrons
* **Reduction:** Gain of electronsIn the context of a battery, the cathode is the site of reduction, where the positively charged ions come to accept electrons. This process generates the flow of electrical current that powers our devices.
As an old battery saying goes, “An electron lost is an electron gained.” Okay, I just made that up, but it does nicely illustrate the intimate connection between oxidation and reduction reactions.
The Role of Electrons in Cathode Operation
Electrons are the lifeblood of any electrical device, and cathodes play a crucial role in managing their flow. As I mentioned earlier, a cathode is a site where reduction occurs. This means that it’s responsible for accepting electrons from the external circuit.
In simple terms, electrons flow from the anode (the site of oxidation) to the cathode (the site of reduction). This flow of electrons creates an electrical current that powers our devices. It’s like a never-ending game of electron tag, with the cathode acting as a home base.
To sum it up, without the efficient management of electrons by cathodes, our devices would be lifeless hunks of metal and plastic. So, the next time you’re enjoying a game on your smartphone or binge-watching your favorite show, take a moment to appreciate the hard-working cathodes behind the scenes. They’re the unsung heroes that keep our modern world powered up and ready for action.
Conclusion
In our electrifying journey through the world of cathodes, we’ve explored their basic definition, the crucial difference between cathodes and anodes, their various applications, and the fascinating science behind their operation. Cathodes are the unsung heroes of our modern world, playing a vital role in powering the devices we rely on every day.
As we come to the end of our adventure, it’s essential to remember that cathodes are just one piece of the complex puzzle that makes up a battery. To truly appreciate the wonder of these energy-storing marvels, it’s worth exploring other aspects of their inner workings, such as how batteries work, the form of energy they store, and the role of rechargeable batteries.
By deepening our understanding of cathodes and their role in the grand scheme of things, we can appreciate the ingenuity of the engineers and scientists who have harnessed the power of electrons to revolutionize our daily lives. From smartphones to electric vehicles, the possibilities are virtually limitless. So, keep exploring, stay curious, and remember to give a little nod of appreciation to the hard-working cathodes that keep our world charged up and ready to go.
FAQ
Is A Cathode Positive Or Negative?
A cathode can be either positive or negative, depending on the type of electrical device it is part of. In primary cells, like batteries, the cathode is positively charged. In other devices like diodes, vacuum tubes, and cathode-ray tubes, the cathode is negatively charged.
What Is The Difference Between A Cathode And An Anode?
A cathode is an electrode from which the conventional current leaves a polarized electrical device, while an anode is an electrode where the current enters the device. In primary cells, the cathode is positively charged, and the anode is negatively charged. Conversely, in other devices like diodes and vacuum tubes, the cathode is negatively charged, and the anode is positively charged.
What Are Cathodes Used For?
Cathodes are used in various applications, including diodes, vacuum tubes, cathode-ray tubes, oscilloscopes, and batteries. They play an essential role in the functioning of these devices by allowing electrons to enter or exit, facilitating the flow of electrical current.
What Is An Example Of A Cathode?
One example of a cathode is the negatively charged electrode in a diode, a semiconductor device that allows current to flow in only one direction. In this case, the cathode allows electrons to exit the diode, ensuring that the current flows in the desired direction. Another example is the positively charged electrode in a battery, which supplies current to the electrical device it powers.
