A secondary battery, also known as a rechargeable battery, is an energy storage device that can be recharged and reused multiple times. It converts chemical energy into electrical energy through reversible chemical reactions, unlike primary batteries that are single-use. Common examples are Li-ion, NiMH, and lead-acid batteries.
As someone who’s been knee-deep in the battery industry for more years than I care to admit, I’ve seen a revolution unfold right before my eyes. From the first time I held a simple alkaline AA, to working with complex Lithium-ion packs, my journey has been nothing short of electric.
Now, imagine a world where batteries aren’t just single-use disposables, but rechargeable powerhouses that keep our devices humming along day in, day out. That’s the realm of secondary batteries – a topic that’s as exciting as a sudden power surge, and thankfully, less dangerous!
According to Markets and Markets, the global secondary battery market size is projected to reach $85.6 billion by 2025. Let me tell you, that’s a whole lot of energy! So strap in, because we’re about to dive deep into the high-powered world of secondary batteries. Trust me, it’s a ride you don’t want to miss!
Definition and Basic Concepts
Those magical little blocks that power our lives. But, what exactly is a secondary battery? I’m glad you asked. In layman’s terms, a secondary battery is the rechargeable version of the Energizer Bunny. It keeps going and going. Technically speaking, it’s a type of electrical battery which can be charged, discharged into a load, and recharged many times. As opposed to a primary battery, which is thrown away after it has been discharged once. They’re like the superheroes of batteries, stepping in to save the day (or at least your device) over and over again.
Now let’s take a look at how primary and secondary batteries stack up against each other:
| Primary Batteries | Secondary Batteries |
|---|---|
| Single-use, disposable | Rechargeable |
| Lower initial cost | Higher initial cost |
| Limited life span | Longer life span |
| Wide temperature range | Temperature sensitive |
| Lower energy density | Higher energy density |
It’s like comparing apples and oranges, or in this case, AA’s and lithium-ions!
Next on our journey is the inner workings of these secondary powerhouses. Imagine a bustling city, with each component playing a crucial role in keeping the lights on. The anode (negative electrode) is like the power plant, where the energy is generated. The cathode (positive electrode) is like the homes and businesses that use that power. The electrolyte? Well, that’s the power grid, carrying energy from the anode to the cathode. And just like a city, everything needs to work in harmony to keep your devices powered up.
“Understanding a battery is understanding energy. It’s understanding the very force that powers life,” says Dr. John Goodenough, the father of the lithium-ion battery. And trust me, he knows a thing or two about batteries.
To help visualize, let’s look at a basic diagram of a battery:
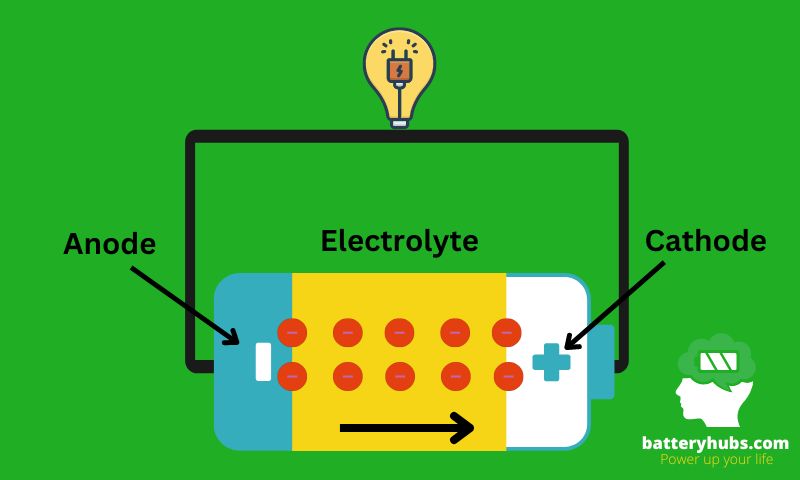
And there you have it. A simple, no-nonsense dive into the fascinating world of secondary batteries. So the next time your phone needs a recharge, you’ll know exactly what’s happening under the hood. Now, isn’t that electrifying?
Deep Dive into Secondary Battery Components
We just went over the basics of what makes a secondary battery tick, but now let’s get our hands a bit dirtier, shall we? It’s time for a deep dive into the heart of these powerhouses: the anode, cathode, electrolyte, and separator. It’s like we’re going on a treasure hunt, and the treasure is knowledge!
The anode, or negative electrode, is where it all begins. This is where the lithium ions are stored and where they return after powering your device. Think of it as the battery’s home base. The anode is typically made of graphite, which, fun fact, is the same stuff that’s in your pencil!
The cathode, or positive electrode, is the destination for our adventurous lithium ions. It’s the city they travel to when your device needs power. The cathode is often made from a material like lithium cobalt oxide. It’s like the anode’s best friend – they can’t function without each other.
The electrolyte plays a crucial role as the highway for the lithium ions. It’s an electrically conductive material that allows ions to move between the anode and cathode. It’s the unsung hero of the battery world.
And finally, the separator. Its job is to prevent the anode and cathode from touching (we don’t want any short circuits), while still allowing the ions to pass through. It’s like a bouncer at a club, keeping the peace while the party goes on.
Let’s see it in a more visual form:
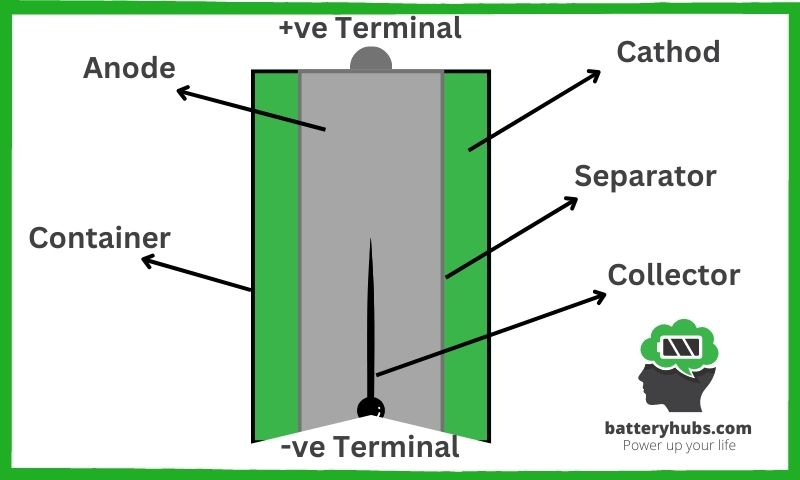
Now, you might be asking yourself, “What’s this ‘positive electrode active material’ you mentioned earlier?” Well, my eager friend, this is the stuff that actually stores the energy in the battery. It’s usually a lithium-based compound in the cathode that gets ‘charged up’ when the battery is charging. It’s like the battery’s secret sauce!
As Dr. Akira Yoshino, the inventor of the lithium-ion battery, once said, “The power of batteries is the power to change the world.” And with a deeper understanding of these components, we’re all a little bit more equipped to harness that power.
Alright, that’s enough of a deep dive for now. Let’s come up for some air and move on to the different types of secondary batteries. It’s a diverse world out there, and we’ve got a lot to cover!
Types of Secondary Batteries
Alright, buckle up! We’re embarking on a thrilling journey through the diverse landscape of secondary batteries. Just like ice cream, they come in many flavors, each with its own unique charm. But today, we’ll be focusing on two crowd favorites: lithium-ion and non-aqueous electrolyte batteries.
Lithium-ion Batteries
Ah, lithium-ion batteries. The rockstars of the battery world. You’ve probably got one in your pocket right now, powering your smartphone! These energy-storing champions are popular due to their high energy density and longevity. They’re like the marathon runners of batteries – they just keep going!
But like anything, they’re not perfect. They can be a little pricey and, if not treated right, can pose safety risks. Remember those hoverboard fires a few years back? Yup, that was due to poor-quality lithium-ion batteries.
Non-aqueous Electrolyte Batteries
On the other side of the ring, we have non-aqueous electrolyte batteries. These are the special ops of batteries, often used in more niche applications like electric vehicles and renewable energy storage. They employ a non-water-based electrolyte, which allows for greater voltage and, consequently, more power.
But, these bad boys can be a bit finicky. They require very specific operating conditions and can be more expensive than their lithium-ion counterparts.
Let’s take a look at how these two stack up:
| Lithium-ion Batteries | Non-aqueous Electrolyte Batteries | |
|---|---|---|
| Advantages | High energy density, Long lifespan | Greater voltage, More power |
| Disadvantages | Higher cost, Potential safety risks | Specific operating conditions, Higher cost |
As you can see, each type of battery has its own strengths and weaknesses. It’s all about choosing the right tool for the job.
“In the world of batteries, it’s a delicate balance between power and practicality,” notes Elon Musk, a major player in the battery-powered electric car industry.
But no matter which type of secondary battery you choose, you’re embracing a technology that’s revolutionizing how we power our world. And that, my friends, is a pretty electrifying thought!
Nickel-Cadmium (NiCd) Batteries
Nickel-Cadmium batteries, or NiCd for short, are the old guard in the battery world. They’ve been around since the early 20th century and were the go-to rechargeable battery for a long time. Durable, reliable, and capable of delivering high discharge rates, NiCd batteries are the rugged workhorses that just won’t quit.
However, NiCd batteries come with their baggage. They suffer from the notorious “memory effect,” which means if they’re not fully discharged before recharging, they may forget their full capacity. Plus, they contain toxic cadmium, which isn’t exactly environmentally friendly.
Nickel-Metal Hydride (NiMH) Batteries
Nickel-Metal Hydride batteries (NiMH) are like the younger sibling of NiCd batteries. They offer a higher energy density and are less harmful to the environment, as they replace cadmium with a hydrogen-absorbing alloy.
Still, NiMH batteries have their quirks. They have a shorter lifespan compared to NiCd and Lithium-ion batteries and can discharge themselves when not in use, which means they might not be ready to go when you are.
Lead-Acid Batteries
Lead-acid batteries are the granddaddies of rechargeable batteries, invented way back in 1859. They’re heavy and not particularly energy-dense, but they’re inexpensive and reliable, which is why they’re commonly used in vehicles for starting, lighting, and ignition (SLI) applications.
The downside? Well, they’re bulky, heavy, and don’t do well with deep discharging. Plus, they contain lead, which isn’t great for the environment.
Let’s bring all this information together in a handy comparison table:
| Nickel-Cadmium (NiCd) | Nickel-Metal Hydride (NiMH) | Lead-Acid | |
|---|---|---|---|
| Advantages | Durable, High discharge rate | Higher energy density, Environmentally friendlier | Inexpensive, Reliable |
| Disadvantages | Memory effect, Toxic cadmium | Shorter lifespan, Self-discharge | Bulky, Poor deep discharge performance, Contains lead |
As Dr. John B. Goodenough, the co-inventor of the lithium-ion battery, wisely said, “Each application needs a different power source.” And this is true when choosing between NiCd, NiMH, and Lead-Acid batteries. Each has its pros and cons, and the choice depends on the specific requirements of the device they’re powering.
Now, with the knowledge of these different types of secondary batteries, let’s dive into the exciting world of battery manufacturing. From raw materials to the final product, it’s a journey you won’t want to miss!
Secondary Battery Manufacturing
Well, folks, we’ve had quite the journey so far, haven’t we? Now, it’s time to roll up our sleeves and delve into the nitty-gritty world of battery manufacturing. If you think it’s all about slapping a couple of metal plates together and calling it a day, boy, are you in for a surprise!
Manufacturing Process for Secondary Batteries
The process of manufacturing secondary batteries is akin to baking a cake. It’s a blend of precise science and a bit of art, with a sprinkle of industrial magic. Here’s a simplified rundown:
- Active Material Synthesis: This is where the active materials for the cathode and anode are prepared. It’s like measuring out the flour and sugar for your cake.
- Electrode Preparation: The active materials are then spread onto metal foils to form the electrodes. Imagine spreading your cake mix into the baking tray.
- Assembly: The anode, cathode, and separator (to prevent short-circuiting) are assembled together, often in a winding process for cylindrical batteries or a stacking process for prismatic (square or rectangular) batteries.
- Formation: The assembled battery is then charged and discharged a few times in a process called “formation” to activate it. It’s like baking your cake to perfection.
- Inspection and Packaging: Finally, the batteries are inspected for quality, packed, and shipped off. Time to enjoy the cake!
Manufacturing Air Secondary Batteries
Air secondary batteries, also known as metal-air batteries, are a special breed. They use oxygen from the air as their cathode, making them lighter and potentially more energy-dense.
Their manufacturing process has a unique twist. Instead of preparing a traditional cathode, an air cathode — a layer that lets in oxygen from the air — is prepared. It’s an intriguing variant of the usual process and one that’s attracting a lot of research interest.
Materials Used in the Manufacturing Process
The materials used in secondary batteries differ depending on the type of battery. For instance, lithium-ion batteries use lithium compounds in their cathodes, while lead-acid batteries use lead dioxide.
Regardless of the type, the materials used must be high purity and precisely prepared to ensure battery performance. As Jeff Dahn, a leading researcher in lithium-ion batteries, once said, “The quality of the materials is the quality of the battery.”
So there you have it, a glimpse into the fascinating world of secondary battery manufacturing. It’s a process of precision, quality control, and a dash of industrial wizardry. And next time you charge your phone, spare a thought for the intricate process that brought that battery to life!
Applications of Secondary Batteries and Energy Storage
After an electrifying journey through the making of secondary batteries, I believe it’s time we talk about where these power-packed marvels actually end up. Ready to explore? Great! Let’s jump right into it.
Use of Secondary Batteries in Various Industries and Everyday Devices
Secondary batteries are like the unsung heroes of our modern life. From the moment you silence your alarm in the morning, to when you’re binge-watching your favorite show late at night, these trusty sidekicks are powering your day.
- Consumer Electronics: This is where most people interact with secondary batteries daily. Your smartphones, laptops, and wearable devices all rely on these powerhouses to keep you connected and entertained.
- Electric Vehicles (EVs): The EV revolution is being fueled, quite literally, by secondary batteries. They provide the juice that drives electric cars, buses, and even some experimental airplanes.
- Medical Devices: Imagine pacemakers, hearing aids, and portable medical equipment. Yes, you guessed it right! All these vital devices are backed by secondary batteries.
- Industrial Applications: Secondary batteries power everything from forklifts to power backups in factories and data centers.
Use of Secondary Batteries in Energy Storage Systems
Now, let’s talk about energy storage systems. These are like giant reservoirs holding onto electricity until it’s needed. And guess what forms the heart of these systems? Bingo! Secondary batteries.
Energy storage systems are critical for smoothing out the supply of electricity from renewable sources like wind and solar, which can be somewhat, let’s say, temperamental. When the sun’s shining or the wind’s blowing, the excess power can be stored. Then, when things calm down, this stored energy is unleashed to keep the lights on.
Case Studies Highlighting Their Importance
To truly appreciate the power of secondary batteries, let’s look at a couple of real-world examples:
- Tesla’s Hornsdale Power Reserve: This energy storage facility in South Australia uses Tesla’s lithium-ion batteries to store energy from a nearby wind farm. It’s capable of supplying power to approximately 30,000 homes for over an hour during peak demand. How’s that for a power move?
- Medical Devices in Remote Areas: In many remote and underprivileged regions, medical devices powered by rechargeable batteries are literally saving lives. They provide crucial health services where reliable electricity isn’t a given.
- Powering Electric Vehicles: The likes of Tesla, Nissan, and Chevrolet have brought electric vehicles mainstream, all thanks to the advancements in lithium-ion battery technology.
So, as we wind down this section, remember that the unsung hero of our modern life – the humble secondary battery – is working tirelessly behind the scenes. Whether you’re scrolling through this on your phone, or reading this during a power outage on your battery-backed laptop, take a moment to appreciate the power at your fingertips. Isn’t it electrifying?
Secondary Battery Pack and Standardization
Having powered through the many aspects of secondary batteries, let’s switch gears and talk about battery packs and the importance of standardization. It’s a lot like cooking, you see. You can’t just toss ingredients together and hope it tastes good. There are recipes to follow, temperatures to maintain, and timings to adhere to. Similarly, in the world of batteries, there are guidelines, standards, and tests that ensure the end product is safe, efficient, and reliable.
What are Secondary Battery Packs?
Let’s imagine you’re hiking. You have a flashlight, but it’s not quite bright enough. What do you do? You add more batteries! Now, imagine these batteries are neatly packed together, working as a team. That’s essentially what a secondary battery pack is – a group of secondary batteries working together to provide more power.
Battery packs are crucial in applications that require more power than a single battery can provide. Think electric vehicles, solar energy storage, and even your laptop, all of which depend on battery packs for their energy needs.
Standardization and Testing Requirements for Secondary Batteries
Now, let’s talk about the rule book – the standardization and testing requirements for secondary batteries. Organizations like the International Electrotechnical Commission (IEC) and Underwriters Laboratories (UL) have set guidelines that secondary batteries must meet. These guidelines cover a range of parameters, including safety, performance, and environmental impact.
Tests include mechanical stress testing (ever accidentally dropped your phone? Thank the stress tests!), thermal tests, and electrical tests, among others. You see, it’s a pretty thorough process, much like the final exams at school, but for batteries.
Importance of Standards in Ensuring Safety and Performance
Why all these tests, you might ask? Well, imagine driving a car without any safety tests. Scary, right? That’s why these standards are crucial. They ensure that the batteries we use every day, from our phones to our cars, are safe and efficient.
These standards help prevent issues like overheating, short-circuiting, and even fires, ensuring that the battery not only performs well but also does so safely. They also help ensure that the performance claims made by manufacturers aren’t just marketing fluff, but are backed by rigorous testing.
So, while it might be a bit of a hassle for the manufacturers, these standards and tests are like the unsung superheroes, ensuring that the batteries we depend on daily are safe, reliable, and efficient. And in the grand scheme of things, I think we can all agree that’s a win-win situation. Batteries might not be the most glamorous topic, but, as you’ve seen, there’s a lot more to these everyday marvels than meets the eye!
The Process of Charging and Discharging
Alright, folks, it’s time to dive into the heart of the action – the electrochemical processes involved in charging and discharging secondary batteries. If you think of the battery as the stage, the charging and discharging processes are the main acts of the show. And trust me, it’s a performance you don’t want to miss!
The Electrochemical Processes of Charging and Discharging
Let’s start with charging. Imagine you’re at a party, and you’re handing out energy drinks (these are our electrons). You’re our anode, and the folks receiving the drinks are the cathode. When you charge a battery, you’re essentially stocking up on these energy drinks at the anode. This is achieved through an external power source that pushes the electrons from the cathode to the anode.
Now, when the party gets started (discharging), you start handing out those energy drinks back to the cathode through the electrolyte, which is our party venue. The energy drinks (electrons) travel through a circuit, powering our devices, until they’re back at the cathode. And voila, that’s electricity!
Charging -- Electrons flow from --> Cathode
Step-01: Cathode -- to --> Anode
Step-02: Anode -- Stocking up on electrons --> Charging
Discharging -- Electrons flow from --> Anode
Step-01: Anode -- to --> Cathode
Step-02: Cathode -- Powering our devices --> Discharging
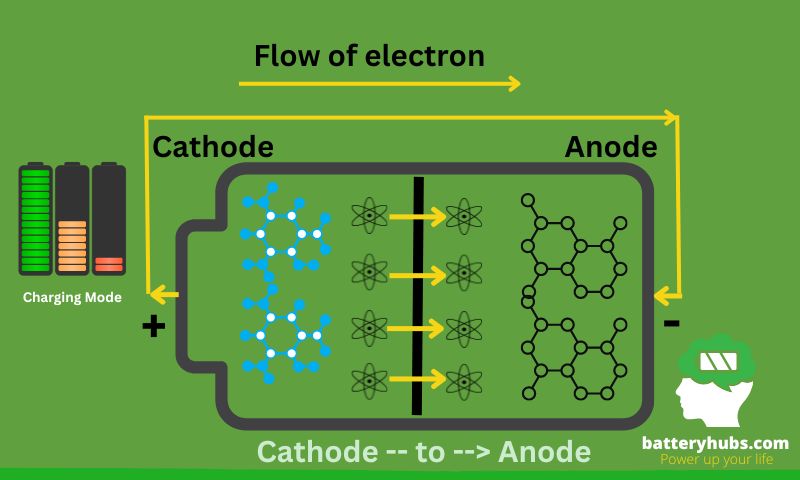
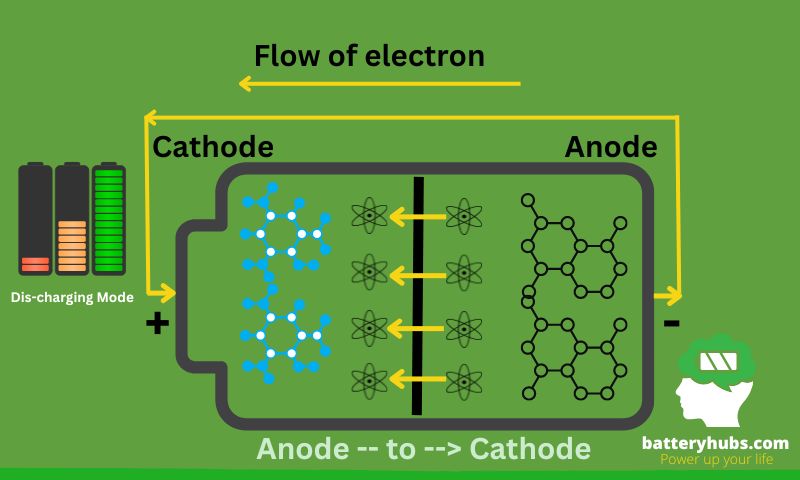
Importance of Understanding These Processes
So, why should you care about these processes? Well, think of it like cooking. If you understand the recipe and the cooking process, you can make the dish more efficiently, and maybe even tweak it to your liking.
Understanding the charging and discharging processes allows us to use our batteries more efficiently, leading to longer battery life. It also helps us identify potential issues, like overheating, before they become a problem. In fact, one of the leading causes of reduced battery life is overcharging, so understanding these processes can help you maintain your battery’s health.
In essence, knowing the ins and outs of these processes is like having a backstage pass to the show. It allows you to appreciate the performance, yes, but it also gives you a chance to influence the outcome. And who wouldn’t want that?
Designing Framework for Secondary Batteries
Now, if you’re anything like me, you’re probably wondering, “How in the world do they design these energy powerhouses?” Well, my friends, designing secondary batteries is a bit like cooking a gourmet meal. You have to consider the ingredients (materials), the recipe (design), and of course, the taste (performance).
Factors Considered When Designing Secondary Batteries
So, what goes into the mix when designing these batteries? A few crucial things, actually.
- Battery Chemistry: The type of chemistry used will significantly impact the battery’s performance, lifespan, and safety. It’s like choosing between baking a cake or grilling a steak – each offers a different outcome.
- Material Quality: The quality of the materials used (anode, cathode, and electrolyte) will directly influence the battery’s efficiency and lifespan. In our culinary analogy, think of it as using fresh, high-quality ingredients instead of old, stale ones.
- Design and Construction: The battery’s design, including the arrangement of the anode, cathode, and electrolyte, can make a big difference in its performance. It’s like plating your dish in a way that’s both functional and pleasing to the eye.
- Safety Measures: Designing for safety is crucial to prevent problems like overheating or short-circuiting. Think of it as following safety guidelines when using a sharp knife or a hot stove.
Impact of Design Choices on Battery Performance and Lifespan
The design choices made when creating a secondary battery can have a significant impact on its performance and lifespan. For instance, choosing lithium-ion chemistry typically offers a high energy density and long lifespan, making it ideal for devices like smartphones and laptops. But this chemistry can also pose safety risks if not properly managed, hence the need for advanced Battery Management Systems (BMS).
On the other hand, a lead-acid battery design might be ideal for high-power applications like car batteries, but it might not offer the same lifespan as lithium-ion designs. Remember, folks, it’s all about choosing the right tool – or in this case, the right battery – for the job.
The bottom line? Designing secondary batteries is like crafting a gourmet meal. It requires careful consideration of various factors to create a product that’s efficient, reliable, and safe. And just like in cooking, the devil’s in the details. So, next time you’re marveling at your smartphone’s battery life, spare a thought for the folks who designed it. They’ve probably been cooking up a storm in the lab!
Properties of Polyethylene Separator in Secondary Batteries
Alright, we’re going to dive into one of my favorite battery components, the humble yet mighty separator. And specifically, we’ll chat about the star of the show, the polyethylene separator.
The Role of Polyethylene Separators in Secondary Batteries
Now, you might think separators are just simple sheets, but oh boy, they are so much more! Let’s think of them as the unsung heroes of our battery universe, working tirelessly behind the scenes to keep things running smoothly.
Polyethylene separators play a crucial role in secondary batteries. Their primary job? To keep the anode and cathode apart to prevent short-circuiting, while still allowing ions to pass through. They are the peacekeepers of the battery world, maintaining order while ensuring the energy flow isn’t interrupted.
How do Polyethylene Separators Contribute to Overall Function and Safety?
So, how do these separators make a difference in the function and safety of our batteries? Let’s dive in!
- Ionic Conductivity: Polyethylene separators allow ions to move freely between the anode and cathode, a crucial aspect of the battery’s operation. Without this, it’s like a rock concert without music – it just doesn’t work!
- Physical Separation: The separator keeps the anode and cathode from coming into direct contact, preventing short circuits. It’s like a bouncer at a nightclub, keeping the peace and ensuring everything runs smoothly.
- Thermal Stability: Polyethylene separators possess a unique feature – they can melt under high temperatures. Now, this might sound like a problem, but it’s actually a safety mechanism. In the event of overheating, the separator melts and blocks ion flow, effectively shutting down the battery and preventing potential damage or hazards.
- Dimensional Stability: These separators do not easily swell or shrink, ensuring they maintain their structural integrity even under strenuous conditions. This stability is key to maintaining the longevity and safety of the battery.
So, there you have it, folks! The polyethylene separator may seem like a simple sheet, but it’s a critical component that contributes significantly to the function and safety of secondary batteries. It truly is a testament to the saying, “Not all heroes wear capes” – some of them are just thin sheets of polyethylene doing their job in our batteries!
Future Trends in Secondary Battery Technology
Alright, folks! Now that we’ve covered the nitty-gritty of secondary batteries, it’s time to put on our futuristic goggles and have a peek into what’s ahead. Hold on to your seats; we’re going on a time travel to the future of secondary battery technology!
Upcoming Innovations and Advancements in Secondary Battery Technology
First off, let’s talk about the innovations that are about to revolutionize our world.
- Solid-state batteries: These are the next big thing on the horizon. Instead of using a liquid or gel electrolyte, solid-state batteries use a solid one. This not only makes them safer (no leaks!) but also allows for higher energy densities. And guess what? They can potentially offer longer battery life and shorter charging times. It’s like giving our batteries a superpower upgrade!
- Lithium-sulfur batteries: Lithium-sulfur batteries promise to deliver much higher energy densities than current lithium-ion batteries. They’re like the high-octane fuel of the battery world! And what’s more, sulfur is abundant and cheap, making these batteries potentially more cost-effective.
- AI and machine learning in battery design: AI isn’t just for self-driving cars or voice assistants. It’s also finding its way into battery technology. Machine learning algorithms can optimize battery designs for performance and longevity, and even predict battery lifespan based on usage patterns. It’s like having a crystal ball for your battery’s future!
Impact of Future Trends on Energy Storage and Consumption
Now, let’s chat about how these developments might change our energy landscape.
With these advanced battery technologies, we can expect to see major improvements in electric vehicles and renewable energy storage. Electric cars will be able to drive further on a single charge and recharge faster. This might be the push we need to finally say “bye-bye” to those pesky range anxieties!
In the renewable energy sector, higher-capacity and more efficient batteries will make it easier to store energy generated from renewable sources. This could help us make the most out of our sunny days and windy afternoons, and make renewable energy a more reliable and mainstream choice.
So, there you have it! A sneak peek into the future of secondary battery technology. It’s an exciting time to be in the battery world, and I can’t wait to see how these advancements will electrify our future. Stay tuned, my fellow battery enthusiasts, the best is yet to come!
Conclusion
Well, my fellow energy enthusiasts, that’s a wrap! We’ve journeyed through the intricate world of secondary batteries, exploring their components, the various types, and how they’re manufactured. We’ve dived deep into their applications, standards, charging processes, and design frameworks, and even taken a closer look at the humble polyethylene separator. Not to mention our exciting foray into the future of battery technology!
As we’ve seen, secondary batteries are more than just power sources. They’re the beating heart of our modern world, powering everything from our smartphones to electric vehicles, and even renewable energy storage systems.
Remember, every time you charge your device, you’re participating in an incredible electrochemical ballet, perfected over decades of scientific progress. And with the promising developments on the horizon, our energy future looks brighter (and more efficient) than ever.
In the words of the famous physicist Richard Feynman, “There’s plenty of room at the bottom.” The journey of exploration and discovery in the battery world is far from over. So, strap in, because the road ahead is electrifyingly exciting!
Surely, the quest for knowledge never truly ends, and there’s so much more to learn in the electrifying world of batteries. If you’re itching to further expand your understanding, feel free to visit our comprehensive guide on the different types of batteries.
As we power down this conversation on secondary batteries, it’s clear that the world of batteries is a vast and complex one. From the intricacies of lead-acid batteries, to the burgeoning potential of aluminum-ion batteries, there’s always more to learn and explore.
Remember, each type of battery, whether it’s a calcium battery, a flow battery, or a vanadium redox battery, has its own unique advantages and applications. So whether you’re harnessing the power of the sun with a home energy storage system, or simply charging your smartphone, there’s a good chance a secondary battery is making that possible.
And who knows? With the rapid pace of innovation in this field, we may soon see zinc-bromine batteries or even zinc-cerium batteries becoming commonplace in our everyday lives.
So keep exploring, keep asking questions, and keep pushing the boundaries of what’s possible in the world of secondary batteries. The future of energy is in our hands (and our devices), and I can’t wait to see where it takes us next. Until next time, stay charged and stay curious. The world of secondary batteries is waiting for you!
