Alkaline batteries have a higher energy density, longer shelf life, and better performance in high-drain devices, while regular (zinc) batteries are more affordable and suitable for low-drain devices. The main difference lies in their electrolyte composition: potassium hydroxide for alkaline and ammonium chloride or zinc chloride for zinc batteries.
With the plethora of battery-powered devices in our daily lives, from remote controls and digital cameras to flashlights and children’s toys, it is no surprise that the global battery market was valued at $126.1 billion in 2020 and is projected to grow at a compound annual growth rate (CAGR) of 7.3% from 2021 to 2028.
Among the various types of batteries, alkaline and regular (zinc) batteries are two of the most common options consumers encounter when purchasing batteries for their devices.
Knowing the difference between these two types of batteries is essential for maximizing the performance and lifespan of your electronic devices, as well as making environmentally friendly and cost-effective choices.
In this blog post, we will provide an in-depth comparison between alkaline batteries and regular batteries, discussing their composition, technical specifications, benefits, and applications, and offering recommendations on how to choose the right battery for your needs.
The Inner Workings of Batteries
Before delving into the differences between alkaline and regular batteries, it’s essential to understand the fundamental concepts behind how batteries work.
This includes the basic components of a dry cell battery, the electrochemical reactions that power them, and the vital role that electrolytes play in battery function.
A. The basic components of a dry cell battery
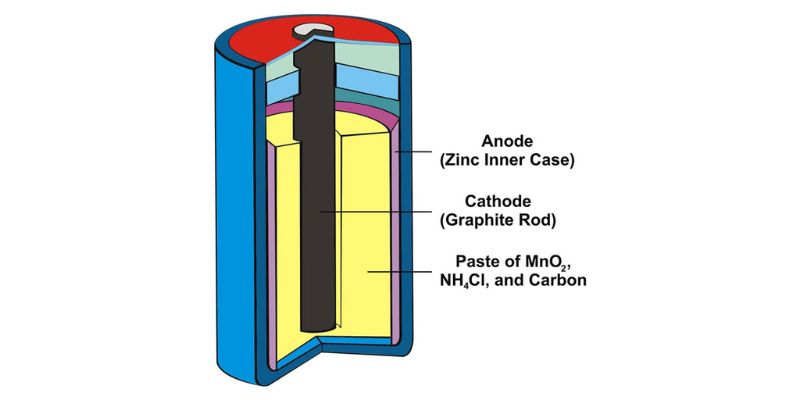
Dry cell batteries, including alkaline and regular (zinc) batteries, consist of three primary components:
- Anode: The anode is the battery’s negative terminal, which is usually made of zinc or another metal.
- Cathode: The cathode is the battery’s positive terminal, typically composed of manganese dioxide or another metal oxide.
- Electrolyte: The electrolyte is the medium that facilitates the flow of ions between the anode and the cathode.
In alkaline batteries, the electrolyte is usually potassium hydroxide, while in zinc batteries, it is typically ammonium chloride or zinc chloride.
B. Electrochemical reactions in batteries
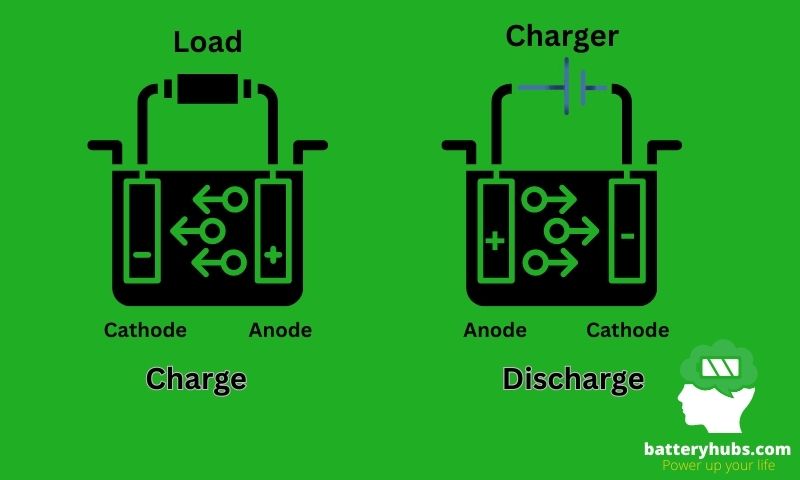
The operation of a battery relies on an electrochemical reaction that occurs between the anode and the cathode. When the battery is connected to a device, a chemical reaction occurs at the anode, causing it to release electrons. These electrons then flow through the device, creating an electric current.
Simultaneously, a chemical reaction occurs at the cathode, where it gains electrons. The electrolyte serves as the bridge that allows ions to flow between the anode and the cathode, balancing the charge and enabling the flow of electricity.
The chemical reaction equations for the electrochemical reactions in an alkaline battery and a zinc-carbon (regular) battery are as follows:
Alkaline Battery:
Anode (Zinc):
Zn(s) → Zn2+(aq) + 2e−
Cathode (Manganese dioxide):
2MnO2(s) + 2e− + 2H2O(l) → 2MnOOH(s) + 2OH−(aq)
Overall reaction:
Zn(s) + 2MnO2(s) + 2H2O(l) → Zn2+(aq) + 2MnOOH(s) + 2OH−(aq)
Zinc-Carbon (Regular) Battery:
Anode (Zinc):
Zn(s) → Zn2+(aq) + 2e−
Cathode (Manganese dioxide):
2MnO2(s) + 2e− + 2NH4+(aq) → Mn2O3(s) + 2NH3(aq) + H2O(l)
Overall reaction:
Zn(s) + 2MnO2(s) + 2NH4+(aq) → Zn2+(aq) + Mn2O3(s) + 2NH3(aq) + H2O(l)
In both batteries, the anode reaction involves the oxidation of zinc, while the cathode reaction involves the reduction of manganese dioxide.
However, the specific reactions at the cathode differ due to the different electrolytes used in alkaline and zinc-carbon batteries.
C. Role of electrolytes in battery function
Electrolytes are crucial for the efficient functioning of a battery. They serve as the conductive medium that allows ions to flow between the anode and the cathode, facilitating the electrochemical reaction.
The choice of electrolyte can significantly impact the battery’s performance, including its energy density, voltage, capacity, and shelf life. Different types of batteries use different electrolytes, which contribute to their unique characteristics.
For example, alkaline batteries use potassium hydroxide as the electrolyte, while zinc batteries use ammonium chloride or zinc chloride. The specific electrolyte used can determine the battery’s overall performance, making it a critical factor in the comparison between alkaline and regular batteries.
Alkaline Batteries: Composition and Technical Specifications
Now that we’ve explored the differences between alkaline and regular batteries, let’s delve deeper into the composition and technical specifications of alkaline batteries, including their chemical composition, physical construction, performance characteristics, and various types and voltage ranges.
A. Chemical composition and electrolyte
Alkaline batteries are named after their alkaline electrolyte, which is potassium hydroxide (KOH) in aqueous solution. The anode in an alkaline battery is made of zinc (Zn), while the cathode is composed of manganese dioxide (MnO2). The combination of these materials and the alkaline electrolyte contributes to the battery’s performance and capacity.
B. Physical construction
The construction of an alkaline battery consists of a cylindrical or rectangular outer casing made of steel or another corrosion-resistant material. Inside the casing, a layer of manganese dioxide surrounds a zinc gel core, which serves as the anode. The potassium hydroxide electrolyte is in contact with both the anode and the cathode, allowing the flow of ions between them.
A separator is placed between the anode and the cathode to prevent them from coming into direct contact, which could cause a short circuit. The battery terminals are connected to the anode and the cathode, allowing the flow of electricity when the battery is connected to a device.
C. Performance characteristics
Alkaline batteries offer several advantages in terms of performance compared to regular zinc batteries. They exhibit a higher energy density, enabling them to store more energy per unit volume or weight.
Another notable performance characteristic of alkaline batteries is their extended shelf life, which can reach 5-7 years, thanks to their lower self-discharge rate. They are also capable of functioning efficiently across a wide range of temperatures, ensuring consistent performance even in extreme conditions.
In addition to these benefits, alkaline batteries are recognized for their low internal resistance, allowing them to deliver high currents, making them well-suited for high-drain devices.
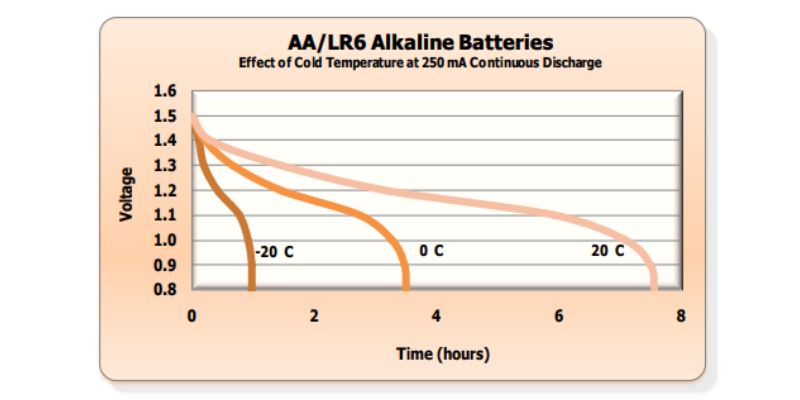
However, the performance of alkaline batteries at temperatures as low as -30 degrees Celsius raises some considerations. Unfortunately, comprehensive information on the effects of alkaline batteries operating at -30 degrees Celsius is not readily available.
While one source suggests that the energy loss in alkaline batteries at lower temperatures (-30 degrees Celsius) appears to be insignificant based on a graph for Duracell AA Coppertop batteries, another source adopts a more cautious viewpoint, particularly for extremely cold temperatures like -30 degrees Celsius. It proposes that the internal resistance of alkaline batteries may increase from 0.2 ohms at room temperature to 0.5 ohms at -30 degrees Celsius, potentially impacting their ability to deliver the required 500mA current.
For low-temperature applications, NiCd (Nickel-Cadmium) batteries are recommended. They have been extensively characterized and documented for operation at temperatures as low as -40 degrees Celsius. NiCd batteries can still deliver a significant portion of their rated capacity (around 25-40%) at -40 degrees Celsius and can accept a charge at low temperatures without sustaining damage.
Silver Oxide batteries are also mentioned as an alternative for low-temperature applications, although they tend to be more expensive compared to alkaline batteries. These batteries may require insulation and increased capacity to cope with cold temperatures.
It is essential to note that alkaline batteries are primary batteries, whereas NiCd batteries are rechargeable (secondary) batteries. The choice between these battery types depends on the specific requirements of the IoT project.
D. Types and voltage range of alkaline batteries
Alkaline batteries are available in various sizes and shapes to suit different devices’ needs. Some common sizes include AAA, AA, C, D, and 9V.
The voltage of a single alkaline battery cell is typically around 1.5 volts, while a 9V alkaline battery contains six 1.5V cells connected in series, giving it a total voltage of 9 volts.
It is essential to choose the appropriate battery type and voltage for the specific device to ensure optimal performance and avoid potential damage.
Regular (Zinc) Batteries: Composition and Technical Specifications
Having examined the intricacies of alkaline batteries, it’s time to shift our focus to regular (zinc) batteries and explore their chemical composition, electrolyte, physical construction, performance characteristics, as well as the types and voltage range they encompass.
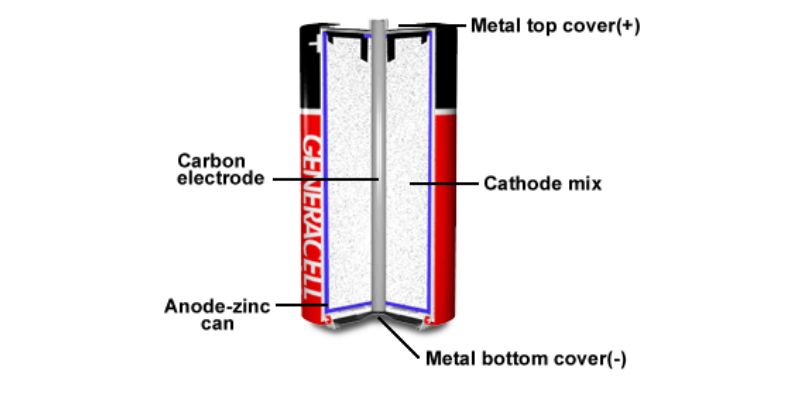
A. Chemical composition and electrolyte
A zinc battery, also known as a zinc-carbon battery or a dry cell, is composed of several important components. Firstly, it features a solid zinc can that functions as the anode of the battery. The cathode is a mixture comprising mainly of moist manganese dioxide powder, carbon black, an electrolyte, and a specially blended solution. A carbon electrode rod is situated in the center of the battery, with the top and bottom covers being metallic, serving as the positive and negative terminals respectively.
The chemical processes within a zinc-carbon battery involve a zinc anode, a manganese dioxide cathode, and an electrolyte that is usually either ammonium chloride or zinc chloride dissolved in water. The cell generates electricity by shifting or modifying equivalent quantities of electrode material salts. The cathode may also contain solid ammonium chloride acting as a fuel reserve during intermittent usage. Additional substances like gum karaya and ion exchange resins might be added to the cathode to boost discharge efficiency.
Another element in a zinc-carbon battery is the separator, which is made from cereal paste and an electrolyte solution and can be up to 3.5 mm thick. This separator functions as both an electrolyte storage and a membrane separating the electrodes.
There are two major categories of zinc-carbon batteries available on the market: cylindrical cells and flat cells. The former can be purchased as single units or in multi-cell packs, whereas the latter is usually available in stacks ranging from four to hundreds of cells.
It’s important to highlight that the manufacture of zinc-carbon batteries necessitates high-quality electrode and packaging materials. In modern dry cells, zinc is often mixed with a minute amount of mercury (less than 1 part per million) to enhance its resistance to corrosion. The zinc may also include about 0.05% cadmium and 0.25% lead to improve grain refinement, hardness, and corrosion resistance. However, the use of cadmium and mercury has been largely discontinued in these batteries made in the United States since 1990 due to environmental disposal concerns.
The cathode material, manganese dioxide, must be very pure, typically sourced from mines in countries such as Mexico, Gabon, China, and Brazil. This substance is mixed with graphite or acetylene black to boost the conductivity and absorption of the electrolyte. While a small quantity of graphite is generally used, the bulk of the carbon comes from acetylene black owing to its stability and high conductivity. The source for this information is the Molecular Expressions website’s section on zinc-carbon batteries.
B. Physical construction
Zinc batteries have a cylindrical or rectangular outer casing made of zinc or another corrosion-resistant material. Inside the casing, a mixture of manganese dioxide and carbon powder forms the cathode, while a zinc canister or gel serves as the anode.
The electrolyte paste is in contact with both the anode and the cathode, allowing for the flow of ions between them. A separator, typically made of porous materials like paper or fabric, is placed between the anode and the cathode to prevent direct contact and short-circuiting. The battery terminals are connected to the anode and the cathode, enabling the flow of electricity when the battery is connected to a device.
C. Performance characteristics
Zinc batteries are generally more affordable than alkaline batteries, but they come with some performance trade-offs. They have a lower energy density and a shorter shelf life compared to alkaline batteries.
Zinc batteries are best suited for low-drain devices due to their relatively high internal resistance, which restricts the flow of high currents. The performance of zinc batteries can be affected by extreme temperatures, making them less versatile compared to alkaline batteries.
D. Types and voltage range of zinc batteries
Zinc batteries are available in various sizes and shapes to cater to the needs of different devices. Common sizes include AAA, AA, C, D, and 9V. The voltage of a single zinc battery cell is typically around 1.5 volts, similar to that of an alkaline battery.
A 9V zinc battery contains six 1.5V cells connected in series, resulting in a total voltage of 9 volts. When selecting a zinc battery, it is crucial to choose the appropriate type and voltage for the specific device to ensure optimal performance and prevent potential damage.
Comparing Alkaline and Regular (Zinc) Batteries
With a deeper understanding of the composition and specifications of both alkaline and regular (zinc) batteries, let’s move on to compare their energy output, efficiency, performance, and pricing to help determine which option provides the best value for money based on various needs and requirements.
| Feature | Alkaline Batteries | Regular (Zinc) Batteries |
| Electrolyte | Potassium hydroxide (KOH) | Ammonium chloride (NH4Cl) or zinc chloride (ZnCl2) |
| Energy Density | High | Low |
| Shelf Life | Longer (5-7 years) | Shorter (1-2 years) |
| Temperature Performance | Operates efficiently in a wide range of temperatures | Performance affected by extreme temperatures |
| Internal Resistance | Low | High |
| Best Suited Devices | High-drain devices (e.g., digital cameras, toothbrushes, game controllers) | Low-drain devices (e.g., remote controls, clocks, torches) |
| Price | Slightly more expensive | More affordable |
| Environmental Impact | Less waste due to longer lifespan | More waste due to shorter lifespan |
Keep in mind that while this table provides a general comparison between alkaline and regular (zinc) batteries, specific battery performance may vary depending on the manufacturer and model. It is essential to consider your device’s requirements and usage patterns when selecting the appropriate battery type.
Benefits and Applications of Alkaline Batteries
As we transition from understanding the composition of alkaline batteries to exploring their benefits and applications, we’ll examine their high energy density, longer shelf life, anti-leak protection, and contact resistance reduction, as well as their suitability for high-drain devices and the environmental advantages they offer.
A. High energy density and longer shelf life
Alkaline batteries offer a higher energy density compared to regular (zinc) batteries, meaning they can store more energy per unit volume or weight. This makes them a superior choice for devices that require extended periods of use or frequent battery replacement.
Additionally, alkaline batteries have a longer shelf life, typically 5-7 years, due to their lower self-discharge rate, ensuring they maintain their charge for longer periods when not in use.
B. Anti-leak protection and contact resistance reduction
Many alkaline batteries feature anti-leak protection, which helps prevent the leakage of electrolytes and potential damage to devices. The design of alkaline batteries also contributes to lower internal resistance, which allows for more efficient power delivery and better performance, especially in high-drain devices.
C. Ideal for high-drain devices
Alkaline batteries are particularly well-suited for high-drain devices, such as digital cameras, toothbrushes, toys, and game controllers, that require a consistent power supply. Their low internal resistance enables them to deliver high currents, ensuring optimal performance and longevity for these types of devices.
D. Environmental benefits
While both alkaline and zinc batteries contribute to environmental waste, alkaline batteries have some advantages due to their longer lifespan. With a longer shelf life and better performance in high-drain devices, fewer alkaline batteries are needed over time compared to zinc batteries, reducing overall waste.
Additionally, many alkaline batteries are now manufactured with reduced mercury content or are mercury-free, further minimizing their environmental impact.
While alkaline batteries offer numerous benefits, regular (zinc) batteries also have their own set of advantages and applications, making them a suitable choice for certain devices and situations.
Benefits and Applications of Regular (Zinc) Batteries
Shifting our attention from alkaline batteries to the benefits and applications of regular (zinc) batteries, we will discuss their simple, reliable technology, economical nature for low-drain devices, excellent price-quality ratio, and the environmental advantages they provide.
A. Simple, reliable technology
Zinc batteries have been in use for many years, providing a simple and reliable technology that has stood the test of time. Their straightforward design and construction make them a tried-and-true option for many basic power needs, offering consistent performance for various everyday devices.
B. Economical for low-drain devices
One of the main advantages of zinc batteries is their cost-effectiveness when used in low-drain devices, such as remote controls, clocks, and torches.
Since these devices do not require high currents, the higher internal resistance of zinc batteries does not significantly affect their performance, making them a more economical option in these applications.
C. Excellent price-quality ratio
Regular (zinc) batteries generally have a lower price point compared to alkaline batteries, making them an attractive option for budget-conscious consumers.
Despite their lower energy density and shorter shelf life, they still offer satisfactory performance for low-drain devices, giving users an excellent price-quality ratio for many basic power needs.
D. Environmental benefits
While zinc batteries generally have a shorter lifespan than alkaline batteries, contributing to more frequent battery waste, some manufacturers are taking steps to minimize their environmental impact.
These efforts may include reducing the use of harmful chemicals or incorporating recycled materials in the battery production process.
It is essential to properly dispose of zinc batteries to minimize their impact on the environment and consider recycling options where available.
Understanding the differences between alkaline and regular (zinc) batteries is only the first step in making an informed decision about which type to use. It is crucial to select the right battery based on specific needs and device requirements.
Making the Right Choice: Recommendations for Battery Use
In order to ensure optimal performance and longevity for your devices, there are several factors to consider when choosing a battery.
A. Matching battery type to device requirements
Always consult the manufacturer’s recommendations for the battery type suitable for your device. High-drain devices, such as digital cameras and game controllers, typically perform better with alkaline batteries due to their low internal resistance and high energy density.
On the other hand, low-drain devices like remote controls and clocks may work efficiently with more affordable zinc batteries.
B. Rechargeable battery options for various users
Rechargeable batteries, such as nickel-metal hydride (NiMH) or lithium-ion (Li-ion), can be a more sustainable and cost-effective option for devices with frequent battery replacement needs.
They are available in various capacities and can be recharged hundreds or even thousands of times, reducing waste and long-term costs.
When considering rechargeable options, evaluate your usage patterns and choose a battery type that best aligns with your specific needs and device requirements.
C. Interchangeability and emergency use considerations
While alkaline and zinc batteries share the same voltage and can often be used interchangeably in low-drain devices, it is essential to understand that their performance may vary.
In emergency situations, having a supply of both alkaline and zinc batteries can be beneficial, as alkaline batteries are more reliable in extreme temperatures and have a longer shelf life.
However, always prioritize using the recommended battery type for your device to ensure optimal performance and prevent potential damage.
Conclusion
In summary, alkaline batteries offer higher energy density, longer shelf life, and better performance in high-drain devices, while regular (zinc) batteries provide a more affordable option for low-drain devices with an excellent price-quality ratio. Both battery types have their respective advantages and applications, making them suitable for different devices and situations.
Selecting the appropriate battery type for your devices is crucial for ensuring optimal performance, longevity, and cost-effectiveness. By considering factors such as device requirements, usage patterns, and environmental impact, you can make informed decisions about which battery type best suits your needs.
To further enrich your understanding of batteries, it’s critical to delve into the underlying mechanisms and classifications. One key distinction to comprehend is between primary and secondary batteries. By visiting our comprehensive guide on primary batteries, you’ll gain a deeper understanding of non-rechargeable batteries, including zinc and alkaline batteries. This knowledge will assist you in recognizing when to use each type, boosting the efficiency and lifespan of your devices.
Moreover, while we’ve outlined the key differences between zinc and alkaline batteries here, there’s more to explore about the specifics of alkaline batteries. They’re often a go-to choice for many high-drain devices due to their superior energy density and long shelf life. Grasping the detailed workings of these batteries will allow you to make even more informed decisions about battery selection.
Ultimately, the goal is to enhance device performance and lifespan, achieve cost-effectiveness, and reduce environmental impact. With the wealth of resources available, including the manufacturer’s guidelines, online forums, customer reviews, and our detailed battery guides, you’ll be well-equipped to make the right battery choices for your specific needs. Always prioritize safety and environmental responsibility when using and disposing of batteries, and remember to consider recycling options where available.
