Batteries store energy as chemical energy, which is the result of electrochemical reactions occurring within the battery. When in use, the stored chemical energy is converted into electrical energy, providing power to devices. This energy conversion process is reversible, allowing for recharging in certain battery types.
Today, I’m thrilled to dive into a question I get asked quite often: What is the form of energy that batteries store energy as? Now, if you’ve been following my blog for a while, you know I have a ton of experience in the battery world, and I’m always eager to share my knowledge. So, let’s get right to it!
In this post, I’ll be discussing the fascinating world of chemical potential energy, which is the secret sauce behind batteries. Trust me, once you grasp this concept, it’s like a light bulb moment (pun intended). So grab a cup of coffee, and let’s explore this electrifying topic together!
Alright, now that we’ve covered the basics, let’s dive deeper into the nitty-gritty of how batteries store energy. Are you ready for a wild ride? Fasten your seatbelts, because we’re about to go full speed into the world of electrochemical devices and energy conversion!
Understanding How Batteries Store Energy
Before we move on, I want to make sure we’re on the same page. Remember that batteries are the energy storage superheroes we rely on to power our devices. They come in various shapes and sizes, from tiny button cells to large lead-acid batteries for cars. Alright, let’s explore how these champions of energy storage work their magic!
First things first, batteries are electrochemical devices. You might be wondering, “What does that even mean?” Well, in layman’s terms, it means that batteries store and release energy through electrochemical reactions. These reactions involve the transfer of electric charges (hello, electrons!) between different chemical species.
At the heart of every battery, you’ll find a special duo: the electrochemical cells. These cells are made up of an anode (negative charges) and a cathode (positive charges). When a battery is connected to an electric circuit, the electrochemical reactions kick in, and we have ourselves some electrical energy to power our devices. Isn’t that electrifying?
Now, let’s talk about the process of converting electric power into chemical energy. This is the core function of batteries, and it’s what allows them to store energy for later use. It’s like a well-prepared squirrel stashing away nuts for the winter.
To break it down, when we charge a battery (think of plugging in your phone), an electric current flows through the external circuit into the battery. This current drives the endothermic reaction within the battery, causing it to store energy in the form of chemical bonds. The energy remains locked away in these bonds until the battery is connected to a device, at which point it’s released as electrical energy to power our gadgets.
So, we’ve learned how batteries convert and store energy, but where does this knowledge come in handy? Believe it or not, it’s used in a wide range of energy storage systems that help us meet our electricity demand.
Take renewable energy sources, for example. Solar energy and wind energy are fantastic, but they’re not always available when we need them. Enter our heroes, the batteries! They can store excess electricity generated by solar panels or wind turbines, ensuring a steady supply of power even when the sun isn’t shining or the wind isn’t blowing.
Batteries play a crucial role in stabilizing the electricity grid, too. They help balance the demand and supply of electricity, reducing the need for fossil fuels and making our energy system more sustainable. Plus, batteries are essential for electric vehicles and portable electronic devices, keeping us connected and on the move.
As much as I love talking about energy storage, it’s time for us to explore the various types of energy within a battery. Batteries might look simple on the outside, but there’s a whole world of excitement happening inside them. So, let’s pop the hood and take a closer look at the action!
Types of Energy in a Battery
Now that we’ve got our hands dirty with the inner workings of batteries, it’s time to focus on the various types of energy they store. From chemical to electrical energy, it’s a rollercoaster ride of science and fun!
I know I’ve mentioned chemical energy a few times already, but let’s get a clear definition of what it really is. Chemical energy is the energy stored in the bonds between atoms and molecules in a substance. This energy can be released and converted into other forms of energy, like electrical energy, through chemical reactions.
Remember the electrochemical reactions we talked about earlier? That’s where the magic happens! When a battery is connected to a device, these reactions kick-start the process of converting stored chemical energy into electrical energy, which powers our beloved gadgets.
Alright, let’s talk about the main components of a battery: the anode and the cathode. These two electrodes are the stars of the show when it comes to electrochemical cells.
The anode, which is the negative electrode, is where the oxidation reaction takes place. This reaction generates negative charges (electrons) that flow through an external circuit to reach the cathode.
On the other side, the cathode is the positive electrode, where the reduction reaction happens. This reaction consumes the electrons coming from the anode, closing the electric circuit and allowing the flow of current.
Together, the anode and cathode make it possible for batteries to store and release energy through electrochemical reactions. Talk about teamwork!
Let’s delve into the exciting world of chemical reactions! When a battery is connected to a device, a series of redox (reduction-oxidation) reactions take place between the anode and cathode. These reactions involve the transfer of electrons, and they’re what drive the flow of electric current through the circuit.
At the anode, oxidation occurs, and electrons are released. These electrons then travel through the external circuit, powering the device. Finally, at the cathode, reduction happens, and the electrons are consumed.
This series of reactions continues as long as the battery is connected to a device, providing a steady stream of electrical energy. Once the battery’s chemical energy is depleted, it’s time for a recharge or a replacement, depending on the battery type (hello, Rechargeable Batteries!).
And there you have it – a whirlwind tour of the different types of energy in a battery, from chemical to electrical. It’s a fascinating world inside these little powerhouses, and I hope you’ve enjoyed exploring it with me!
Let’s keep the energy flowing and move on to a thrilling topic that’s bound to spark your interest: potential and kinetic energy in batteries. Strap in, because we’re about to embark on an electrifying adventure through these two essential energy forms!
Potential and Kinetic Energy in Batteries
As we dive into the exciting world of potential and kinetic energy in batteries, it’s essential to understand these concepts and how they relate to our favorite energy storage devices. Ready? Let’s get started!
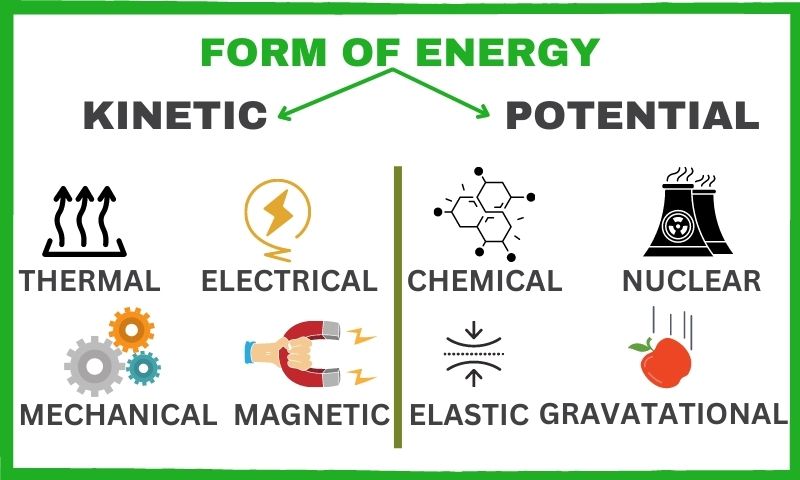
potential energy
Potential energy is the stored energy an object possesses due to its position or state. It’s like having a hidden superpower, just waiting to be unleashed! In the context of batteries, potential energy is the energy stored within the chemical bonds of the battery’s components.
Here’s an example: think of a ball sitting at the top of a hill. It has potential energy because of its position relative to the ground. Similarly, a battery has potential energy due to the chemical bonds between its components that can be converted into electrical energy when needed.
kinetic energy
On the flip side, kinetic energy is the energy of an object in motion. It’s the energy that powers our gadgets, lights our homes, and keeps our lives buzzing with excitement.
Take a battery-powered toy car, for instance. When the car is in motion, the energy driving it is kinetic energy. The battery’s stored potential energy has been converted into kinetic energy to get the car moving.
the difference between potential and kinetic energy in relation to batteries
Now that we know what potential and kinetic energy are, it’s time to see how they relate to batteries. Remember, potential energy is stored energy, and kinetic energy is the energy of motion.
When it comes to batteries, potential energy is the energy stored in the chemical bonds within the battery. This energy remains dormant until the battery is connected to a device. Once connected, a chemical reaction occurs, and the potential energy is converted into kinetic energy in the form of electrical energy. This electrical energy flows through the circuit, powering the device.
In a nutshell, potential energy is the battery’s stored power, while kinetic energy is the electrical energy that gets things moving. Batteries are like a superhero duo, with potential energy as the secret identity and kinetic energy as the heroic alter-ego that saves the day!
And there you have it – a thrilling exploration of potential and kinetic energy in batteries. These dynamic energy forms work hand in hand to power our world, and I hope you’ve enjoyed discovering their secrets with me!
Fasten your seatbelts and hold onto your hats, my fellow energy enthusiasts! We’re about to embark on a fantastic voyage into the world of chemical energy storage in batteries. Get ready to explore the marvels of electron exchange, endothermic reactions, and the amazing applications of chemical energy storage!
Exploring the Chemical Energy Storage Process in Batteries
Our exploration starts with a closer look at the fascinating processes that occur within batteries. Let’s dive into the details and unravel the mysteries of chemical energy storage!
the process of electron exchange
The heart of chemical energy storage in batteries lies in the process of electron exchange through oxidation and reduction, also known as redox reactions. I know, it sounds like a dance between atoms – and it sort of is!
In a redox reaction, one element loses electrons (oxidation) while another gains them (reduction). In a battery, this electron exchange happens between the anode and cathode. The anode undergoes oxidation, losing electrons, while the cathode undergoes reduction, gaining electrons. This dance of electrons is what generates the electric current that powers our devices!
the goal of storing excess heat through endothermic reactions
Now, let’s discuss endothermic reactions. These fascinating reactions absorb heat from their surroundings, storing excess thermal energy in the process. “Why is this important?”, you may ask. Well, storing excess heat helps maintain a stable temperature within the battery, preventing overheating and prolonging the battery’s life. As someone who’s had their fair share of battery-related mishaps, I can tell you that temperature management is crucial for a safe, reliable energy storage system!
the various applications of the chemical energy storage process
Finally, let’s talk about the incredible applications of chemical energy storage in batteries. From the smallest button cell in a hearing aid to the gigantic lithium-ion batteries used in electric vehicles, batteries are everywhere! They power our smartphones, laptops, electric scooters, and even renewable energy systems like solar panels and wind turbines. In fact, large-scale batteries help stabilize the electrical grid, storing excess energy during periods of low demand and releasing it during peak times. Isn’t it amazing how the dance of electrons in batteries impacts our daily lives in so many ways?
So, there you have it! We’ve explored the fascinating world of chemical energy storage in batteries, diving into the electron exchange process, endothermic reactions, and the applications that make our lives easier and more sustainable. As an energy aficionado, I can’t help but be amazed by the power and potential of batteries, and I hope you feel the same way too!
Final Words
Well, my fellow battery enthusiasts, our electrifying journey has come to an end. I hope you’ve enjoyed learning about the incredible world of batteries as much as I have enjoyed sharing my passion and knowledge with you. It’s truly amazing how these tiny (or sometimes not-so-tiny) energy storage devices have revolutionized our lives and paved the way for a more sustainable future.
Remember, the magic of batteries lies in the delicate balance of chemical and physical processes that convert energy from one form to another, powering the devices we rely on every day. Whether it’s a smartphone, an electric car, or a renewable energy system, batteries are at the heart of modern technology.
As you continue to explore the wonders of batteries, I encourage you to stay curious, keep learning, and always appreciate the energy that powers our world. Until next time, happy charging!
As we wrap up our discussion, don’t forget that there’s so much more to explore in the realm of batteries. If you’re interested in diving even deeper, our website has a wealth of resources and articles that cover a wide range of battery-related topics. You can learn about the fascinating process of how batteries work, the journey of electricity traveling from a battery, and the mechanics of batteries in a circuit.
For those of you who want to know more about rechargeable batteries, we’ve got you covered! Check out our articles on how rechargeable batteries work, how they get recharged, their ability to retain charge, and how long they last when in use.
Curious about specific types of batteries, such as the AA battery? Our article on how AA batteries work provides an in-depth explanation. And if you’ve ever wondered why these powerhouses are called “batteries,” our article on the origin of the term is a must-read!
So go ahead, embark on your own battery adventure, and expand your knowledge with these fantastic resources. Happy reading and keep the energy flowing!
FAQ
What Is The Energy Density Of Lithium-Ion Batteries, And How Does It Compare To Other Types Of Batteries?
The energy density of lithium-ion batteries is generally between 150 to 200 watt-hours per kilogram (Wh/kg). This is higher than other common battery types, such as lead-acid batteries (30-50 Wh/kg) and nickel-metal hydride batteries (60-120 Wh/kg). This means that lithium-ion batteries can store more energy in a given volume or mass, making them a popular choice for portable electronics and electric vehicles.
How do endothermic reactions help store excess heat in batteries?
Endothermic reactions are chemical processes that absorb heat from their surroundings. In the context of batteries, certain endothermic reactions can be harnessed to store excess heat generated during charging or discharging. By absorbing and storing this heat, endothermic reactions can help prevent thermal runaway and improve the overall safety and efficiency of the battery.
How long do rechargeable batteries typically last when in use?
The lifespan of a rechargeable battery when in use depends on its type, capacity, discharge rate, and the specific device it powers. For example, a lithium-ion battery in a smartphone may last anywhere from 8 to 48 hours on a single charge, depending on usage patterns. Generally, rechargeable batteries can provide hundreds to thousands of charge cycles before their capacity significantly decreases.
What are some common applications of chemical energy storage in batteries?
Chemical energy storage in batteries is widely used in a variety of applications, including powering portable electronics (e.g., smartphones, laptops), electric vehicles (EVs), grid energy storage, backup power systems, and renewable energy integration (e.g., storing solar or wind-generated electricity for later use).
What is the difference between potential and kinetic energy in relation to batteries?
In the context of batteries, potential energy is the stored energy within the battery’s electrochemical cells, which is released when the battery is connected to an external circuit. Kinetic energy, on the other hand, is the energy of motion. When a battery powers a device, the electrical energy (converted from potential energy) is transformed into various forms of kinetic energy, such as the mechanical energy that drives a motor or the thermal energy generated by electronic devices during operation.
